 J Clin Aesthet Dermatol. 2022;15(2):40–43.
J Clin Aesthet Dermatol. 2022;15(2):40–43.
by; Marco Antonio Munia, MD, PhD; Christine Guarnieri Munia, MD; Meire Brasil Parada, MD; Jose Ben-HurFerraz Parente, MD, PhD and Nelson Wolosker, MD, PhD
All authors are with Hospital Moriah in São Paulo, Brazil.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
Facial injections with hyaluronic acid (HA) dermal fillers have become increasingly common. Hyaluronic acid is currently the most frequently used dermal filler. When compared to collagen for the treatment of nasolabial folds, HA not only produces similar cosmetic results with smaller doses but also lasts longer. Our objective was to evaluate the results of 10 patients with vascular complications associated with HA fillers treated with ultrasound-guided injection of hyaluronidase (HYAL) according to the Brazilian Society of Dermatology guidelines. Admission clinical evaluation revealed that the most frequent signs were: livedo reticularis (100% of the patients), hypoesthesia (50%) and local pain (20%). Although 80% of the patients complained of local pain during HYAL injection, none of them persisted with pain after the procedure ended. The total dose of injected HYAL per patient ranged from 300 to 750 IU (mean 500 IU). Post-HYAL treatment Doppler ultrasound showed pervious facial arteries and veins in 100% of the patients.
Keywords: Hyaluronic acid, ultrasound, complications, fillers
Facial injections with hyaluronic acid (HA) dermal fillers have become increasingly common. According to the American Society of Plastic Surgery 2014 Statistic Report,1 whereas surgical procedures have decreased by 12 percent in 14 years, minimally invasive cosmetic procedures have grown by 154 percent. Similarly, the use of hyaluronic acid facial fillers has increased 253 percent in the same period.
Hyaluronic acid is currently the most frequently used dermal filler. When compared to collagen for the treatment of nasolabial folds, HA not only produces similar cosmetic results with smaller doses but also lasts longer.2
Nevertheless, some complications may arise with the use of HA dermal fillers. Vascular occlusion is the most concerning complication of all, caused either by intravascular injection (arterial or venous), external compression (mostly of the veins), thrombosis or embolization. As a result, blood flow may be interrupted in the occluded facial arteries, leading to ischemia, necrosis3 or ulcerations.4,5 Although they may arise in any area of the face treated with HA, ulcerations are most likely to occur in the glabella6 because of the facial vascular anatomic features.7 On the other hand, necrosis in the nasolabial folds, albeit less common and superficial, may cause disfigurement and thus is potentially disastrous to the patient who seeks aesthetic treatment of facial folds with HA fillers.
Our objective was to evaluate the results of 10 patients with vascular complications associated with HA fillers treated with ultrasound-guided injection of hyaluronidase (HYAL) according to the Brazilian Society of Dermatology guidelines.
Methods
In this retrospective study, we studied 10 patients with various degrees of facial ischemia caused by HA dermal filler injections performed between January 2016 and May 2017.
Patients were evaluated at admission for the presence of livedo reticularis, pustules, and loss of sensitivity in their facial skin. Initial Doppler ultrasound assessment was performed to locate the regional arterial branch and evaluate blood flow in all its extension. Absence of visible blood flow and the presence of tardus parvus were considered signs of possible sites of arterial obstruction.
Indications for treatment and the recommended maximum doses of hyaluronidase followed the Brazilian Society of Dermatology guidelines.8 Treatment was initiated between 3 to 72 hours after HA injection (mean 18 hours).
Hyaluronidase (1500 IU/ 10mL of saline solution) was injected in the affected skin areas with 25-gauge cannula, at 300 to 750 IU doses. Ultrasound evaluation was repeated five minutes after HYAL injections to assess arterial blood flow and/ or reappearance of venous blood flow. HYAL injections were repeated when necessary and the procedure was ended upon ultrasound visualization of both adequate arterial and venous blood flow. Patients had their total injected HYAL dose calculated and were also evaluated for improvement of the initial clinical signs.
Results
Admission clinical evaluation revealed that the most frequent signs were: livedo reticularis (100% of the patients), hypoesthesia (50%) and local pain (20%).
Although 80 percent of the patients complained of local pain during HYAL injection, none of them persisted with pain after the procedure ended. The total dose of injected HYAL per patient ranged from 300 to 750 IU (mean 500 IU).
Post-HYAL treatment Doppler ultrasound showed pervious facial arteries and veins in 100% of the patients. Figures 1 to 3 show Doppler ultrasound images during and after HYAL injections.
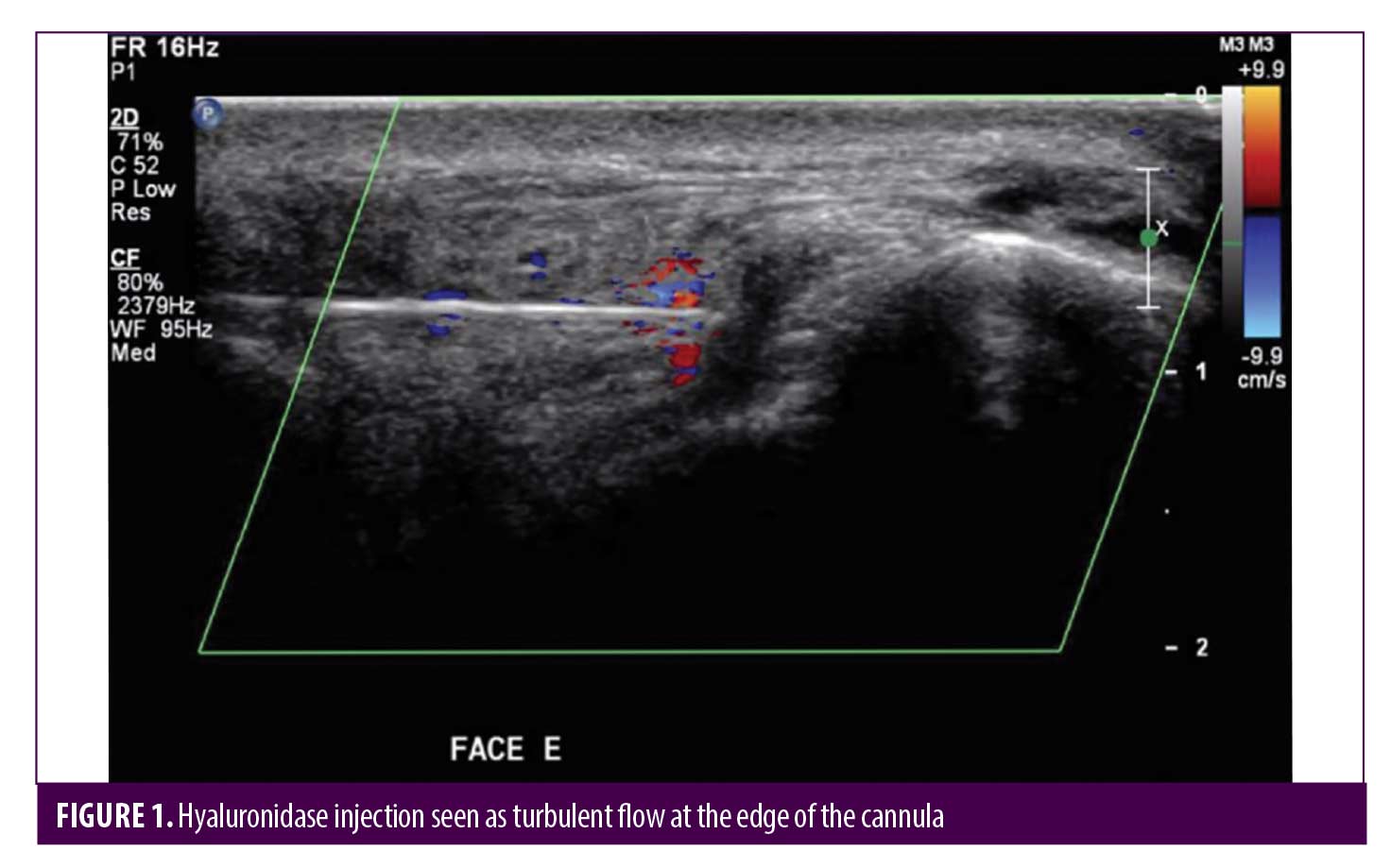
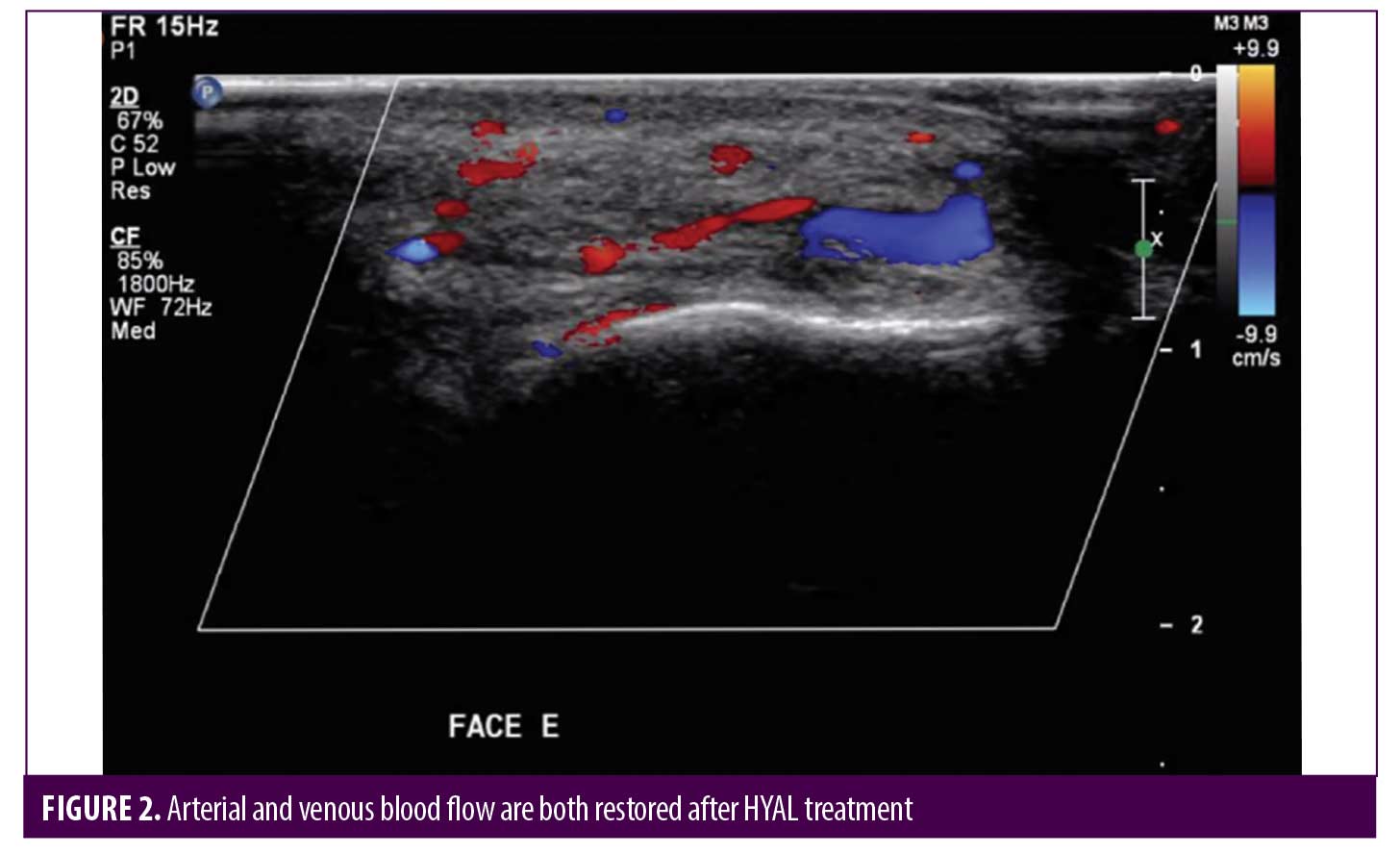
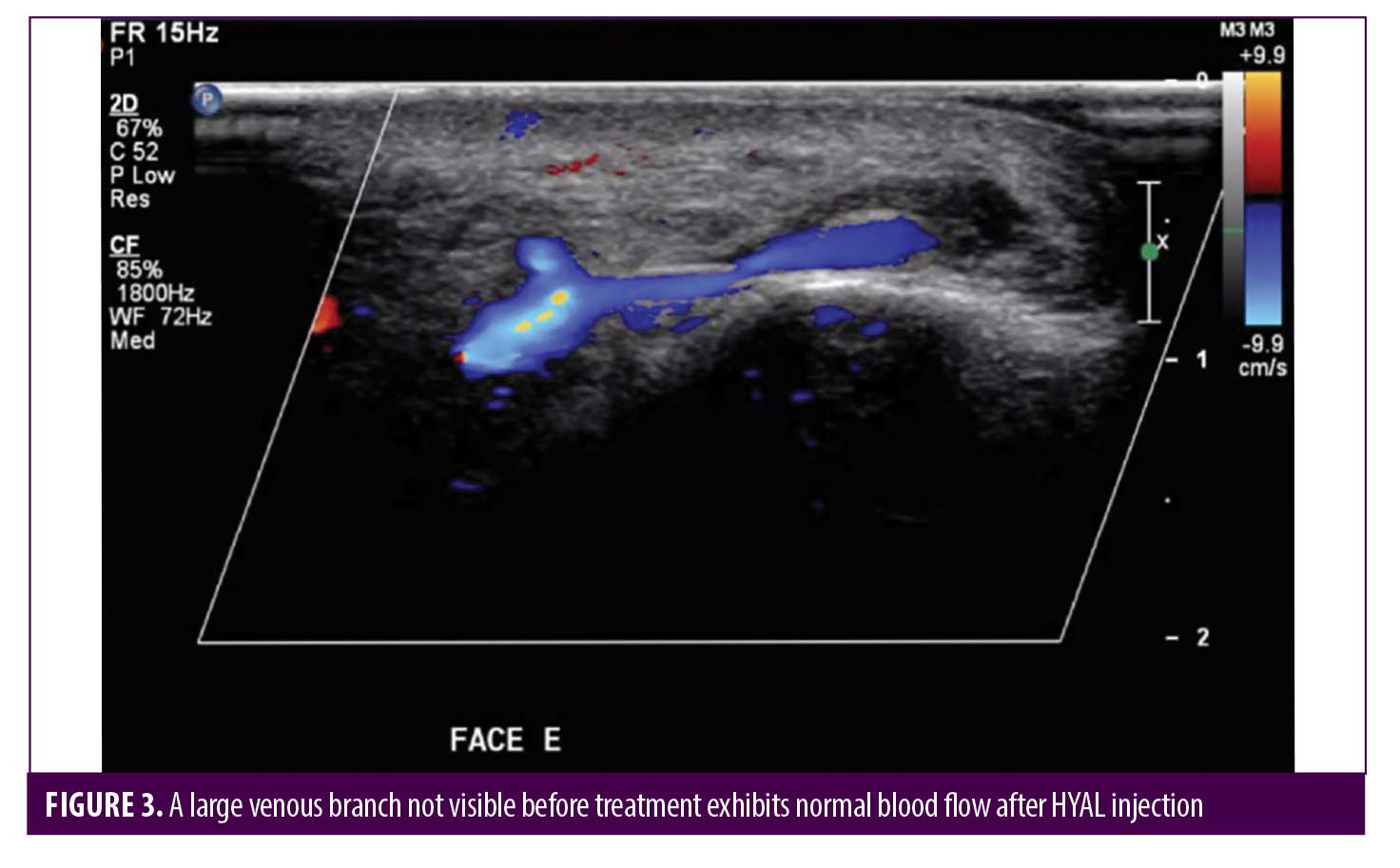
Two patients were additionally treated with Prostavasin® (alprostadil) for 72 hours due to intense livedo reticularis which extended to the nasal ala. Mean time for complete reversal of ischemia was 72 hours (maximum of 7 days). None of the patients had ulcerations, crusts or any other signs of damage to the skin. Figures 4 to 7 show these two patients’ improvement after HYAL treatment.
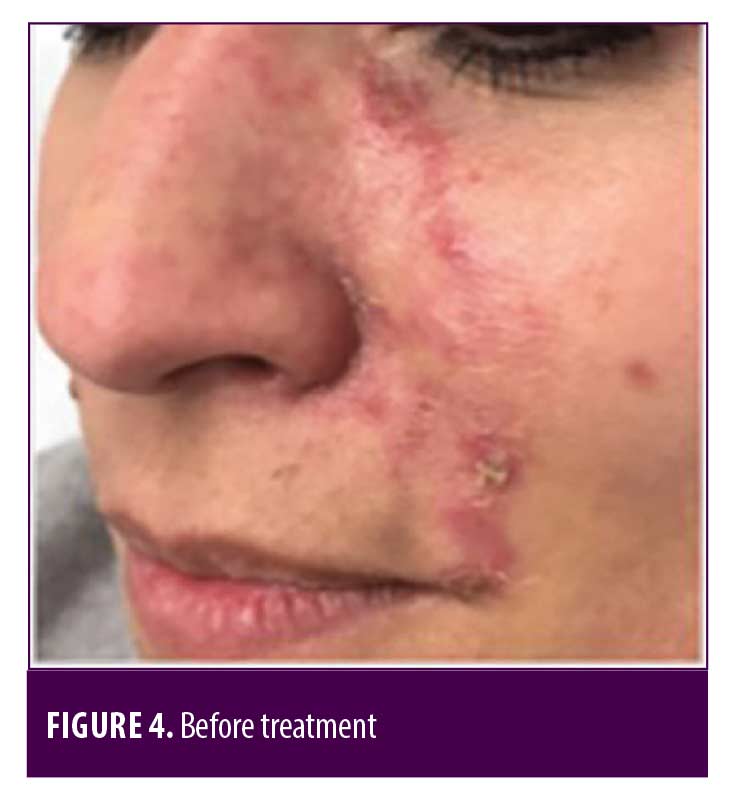
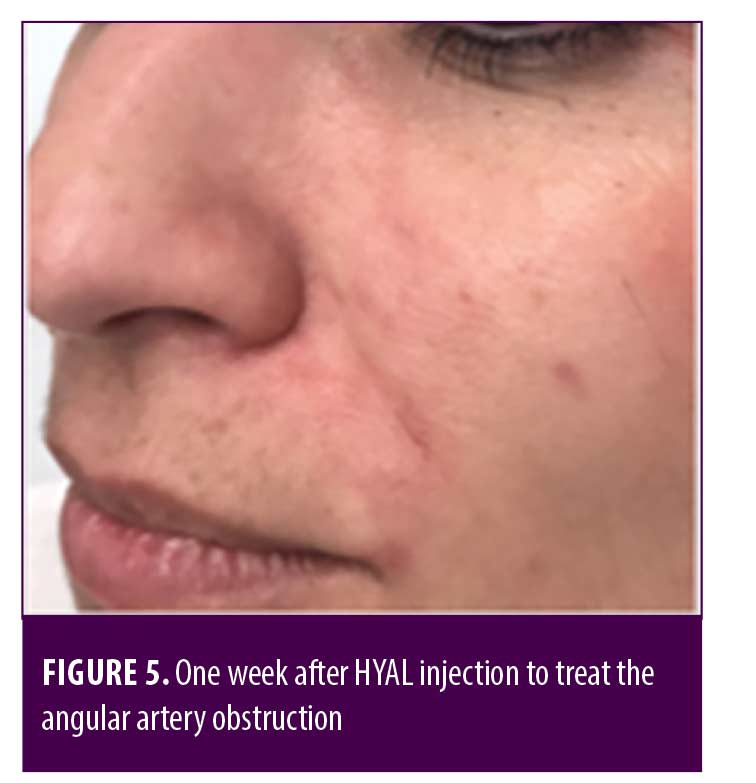
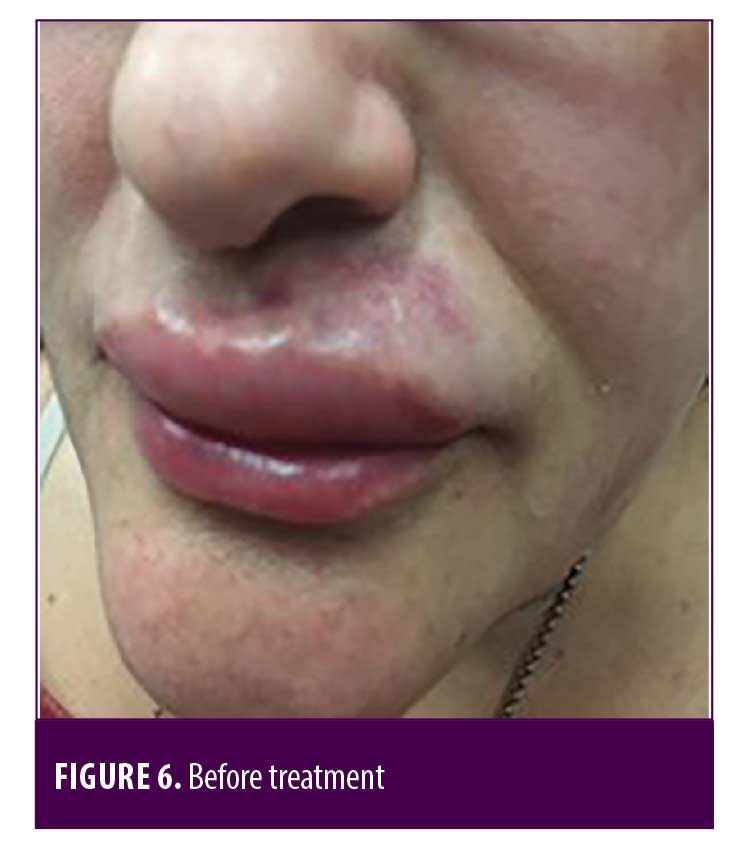

Discussion
Impending skin necrosis is a possible complication associated with all dermal fillers, with an estimated rate of 0.001% of all procedures. The main affected areas are the nose (33.3%) and the nasolabial folds (31.2%). Blindness is mainly related to injections at the glabella (50% of the cases). Severe complications have an overall estimated incidence rate of 0.0001% after HA treatment9.
According to DeLorenzi et al,11 low volume emboli (up to 0.1mL of HA) are the most likely to occur, even by the hands of expert physicians.
Up to date, no standardized treatment is available for post-HA injection ischemia.10 Topical nitroglycerin and oral medications such as salicylic acid or sildenafil have shown limited effectiveness.11
Hyaluronidase, on the other hand, is perhaps the most effective treatment for vascular complications, because it can degrade HA that causes external compression of the blood vessels. In 2014, DeLorenzi11 compared HYAL (300 IU) and saline injections (2mL) to evaluate HA degradation in cadaveric facial soft tissue. Complete HA degradation was demonstrated 4 hours post-HYAL injections whereas no change was observed with saline injections.12
So far, there are no available reports on the use of color Doppler ultrasound to guide HYAL injections directly to the sites of vascular compression or obstruction. By means of Doppler ultrasound, smaller doses of HYAL can be employed to achieve satisfactory results. In addition, ultrasound-guided HYAL injections are more precise and therefore the procedure becomes less painful to the patient.
This technique also allows real-time assessments of blood flow before, during and after treatment, indicating its effectiveness or the need for further HYAL injections to restore vascular permeability.
As described in the study by DeLorenzi,11 no adjuvant topical treatment was applied in this study. The two patients with more severe ischemia of the nasal ala were additionally treated with intravenous vasodilators, which is the established recommendation for critical ischemia of the limbs as well as the face.10,13
A recent 2017 consensus on the prevention and treatment of dermal filler complications recommends the use of cannula and emphasized the importance of applying negative pressure during needle insertion in critical areas of the face.14 Therefore, to minimize the risk of vascular complications with filler injections, areas with critical anatomic features should be avoided and continuous aspiration should be employed during insertion of the cannula or needle. At high risk areas, a 25-gauge cannula is recommended. The dermal filler should be slowly injected, at low pressure, and excessive doses should be avoided. The physician should pay special attention to any sign of pain or changes in skin color (blanching or livedo reticularis).12
Several researchers recommend salicylic acid and repeated high doses of hyaluronidase injected in the affected area until some improvement in capillary perfusion is perceptible.15
We believe that external venous compression may be responsible for the facial ischemia. As in phlegmasia cerulea dolens seen in deep-vein thrombosis, external compression may compromise venous outflow, which retrogradely raises capillary pressure and reduces blood flow in arterioles and arterial capillary vessels, ultimately leading to ischemia and gangrene.16 Doppler ultrasound allows treatment of the exact site of obstruction and direct visualization of the restored blood flow, which increases safety of the aesthetic procedure and minimizes the risk of severe complications.
In his latest study with a HYAL treatment protocol, DeLorenzi described in some patients (particularly the ones with darker skin) the occurrence of blisters, crusts, scarring and/or slight dyschromia. These occasional complications were more intense in patients with multiple ischemic areas in the face, as opposed to those with only one affected area, such as isolated upper lip ischemia. It was suggested that the cause could be insufficient HYAL dose injected in those patients. The author described a “HYAL flooding hypothesis”, i.e., the HA obstructed arteries would have to be flooded by surrounding high doses of HYAL for longer periods for the dermal filler to be dissolved. When additional areas of the face are involved, higher doses of HYAL should be injected.14 Whether arterial or venous, HA obstructed vessels should be bathed by HYAL at concentrations that are high enough to allow it to permeate the vascular wall and decompose HA.
Unlike other researchers, we employed Doppler ultrasound that enabled us to assess all vascular branches, particularly the arterial vessels, and thus inject HYAL directly in the obstruction sites and visualize blood flow restoration. Doppler ultrasound was also helpful to guide the need for repeated injections, as well as to control with greater precision the dose of injected HYAL, therefore diminishing the risk of complications related to insufficient doses and avoiding patients’ complaints related to excessive amounts of HYAL, such as local pain.
Conclusion
Doppler ultrasound can have an important and positive role in the treatment of vascular complications caused by HA fillers. It provides important information on the effects of treatment on local circulation. A low-risk procedure, Doppler ultrasound is also related to better adjustment of treatment doses, greater comfort to the patient, reduced rate of complications and sequelae.
References
- American Society of Plastic Surgeons. ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. 2014 Plastic Surgery Statistics Report. Available at: www.plasticsurgery.org (last accessed 20 May 2016)
- Kontis TC. Contemporary review of injectable facial fillers. JAMA Facial Plast Surg 2013;15: 58–64.
- Hirsch R, Carruthers J. Successful management of an unusual presentation of impending necrosis following a hyaluronic acid injection embolus. Presented at the Annual American Society for Dermatologic Surgery, Palm Desert, CA, Oct 29, 2006.
- Hirsch RJ, Lupo M, Cohen JL, Duffy D. Delayed presentation of impending necrosis following soft tissue augmentation with hyaluronic acid and successful management with hyaluronidase. J Drugs Dermatol 2007;6(3):325–328.
- Lowe NJ. Arterial embolization caused by injection of hyaluronic acid (Restylane). Br J Dermatol 2003;148(2):379; author reply 379–380.
- Edwards PC, Fantasia JF. Review of long-term adverse effects associated with the use of chemically-modified animal and nonanimal source hyaluronic acid dermal fillers. Clin Interv Aging 2007;2(4):509–519.
- Hirsh RJ, Cohen JL, Carruthers JD. Successful management of an junusual presentation of impending necrosis following a hyaluronic acid injection embolus and a proposed algorithm for management with hyaluronidase. Dermatol Surg. 2007;33(3):357–360.
- Parada MB, Cazerta C, Afonso JPJM, Nascimento DIS. Overview and management of fillers complications. Surg Cosmet Dermatol 2015;7(4):332–351.
- Ozturk CN, Li Y, Tung R, Parker L, Piliang MP, Zins JE. Complications following injection of soft-tissue fillers. Aesthet Surg J. 2013;33(6):862–77, 2013.
- Oh BL, Jung C, Park KH, Hong YJ, Wooa SJ. Therapeutic intra-arterial hyaluronidase infusion for ophthalmic artery occlusion following cosmetic facial filler (hyaluronic acid) injection. Neuroophthalmol 2014;38(1):39–43.
- DeLorenzi C. New High Dose Pulsed Hyaluronidase Protocol for Hyaluronic Acid Filler Vascular Adverse Events. Aesthet Surg J. 2017;37(7):814–825.
- DeLorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014 Aug;40(8):832–41.
- Lawall H, Pokrovsky A, Checinski P, Ratushnyuk A, Hamm G, Randerath O, Grieger F, Bentz JW. Efficacy and Safety of Alprostadil in Patients with Peripheral Arterial Occlusive Disease Fontaine Stage IV: Results of a Placebo Controlled Randomised Multicentre Trial (ESPECIAL). Eur J Vasc Endovasc Surg. 2017 Apr;53(4):559-566.
- Philipp-Dormston WG, Bergfeld D, Sommer BM, G Sattler, Cotofana S, Snozzi P, Wollina U, Hoffmann KPJ, Salavastru C, Fritz K. Consensus statement on prevention and management of adverse effects following rejuvenation procedures with Hyaluronic acid based fillers. J Eur Acad Dermatol Venereol. 2017;31(7): 1088–1095.
- DeLorenzi C. Transarterial degradation of hyaluronic acid filler by hyaluronidase. Dermatol Surg. 2014;40(8):832–841.
- Yoshida RA, Sobreira ML. Trombose venosa profunda e embolia pulmonar. In: Wolosker, Nelson. Cirurgia Vascular e Endovascular: abordagem prática/ Nelson Wolosker, Alexandre Fioranelli, Antonio Eduardo Zeratti. 1st ed. Rio de Janeiro: Atheneu, 201.

