 J Clin Aesthet Dermatol. 2018;11(12):21–25
J Clin Aesthet Dermatol. 2018;11(12):21–25
by Lily Jiang, PhD; Peter D. Hino, MD, FAAD; Ashish Bhatia, MD, FAAD; Thomas J. Stephens, PhD; and Felipe Jimenez, Phd
Drs. Jiang, Hino, and Stephens are with Thomas J. Stephens & Associates in Richardson, Texas. Dr. Bhatia is Associate Professor of Clinical Dermatology in the Department of Dermatology at Northwestern University Feinberg School of Medicine in Chicago, Illinois and Director of Dermatologic, Laser & Cosmetic Surgery at Oak Dermatology in Itasca, Illinois. Dr. Jimenez is with Envy Medical Inc. in Long Beach, California.
FUNDING: This study was funded by Envy Medical Inc.
DISCLOSURES: Dr. Bhatia is director and clinical advisor at Envy Medical Inc. and Dr. Jimenez is an employee of Envy Medical Inc. The other authors have no conflicts of interest relevant to the content of this article.
Abstract: Background.Melasma is a common, persistent disorder of hyperpigmented facial skin predominantly attributed to ultraviolet light exposure, hormonal influences, and genetic predisposition.
Objectives. This double-blind, placebo-controlled, randomized clinical trial was conducted to assess the efficacy and tolerance of a multimodality night cream when used over a course of 24 weeks followed by a four-week regression in female subjects with moderate to severe melasma, presence of solar lentigines, and periocular lines and wrinkles.
Methods.Subjects were randomized into one of two groups: Cell 1 received Trifecting® Night Cream (Envy Medical, Long Beach, California) 1.0 and Cell 2 did not. All subjects were supplied with a two-product regimen comprising a cleanser and sunscreen to use during the trial. Clinical grading, tolerability assessments, and Chroma Meter measurements (Konica Minolta, Tokyo, Japan) were performed at baseline and at Weeks 8, 16, 24, and 28 (regression). Standardized digital photographs were taken and self-assessment questionnaires were completed.
Results. Twenty-five subjects completed the 28-week study, with 14 subjects in Cell 1 and 11 subjects in Cell 2. Subjects in both groups showed improvements in facial conditions. Cell 1 outperformed Cell 2 in improving fine lines, solar lentigines, and melasma conditions. These improvements were sustained during regression period.
Conclusions. Trifecting® Night Cream 1.0, is effective for the treatment of moderate to severe melasma, solar lentigines, and periocular lines and wrinkles over 24 weeks of usage, with its benefits sustained for at least four weeks after treatment.
Keywords: Photodamage, melasma, solar lentigines, hyperpgimentation, skin brightening, lumixyl, retinol, antioxidant
Introduction
Melasma is a pigmentary disorder that is caused by a dysfunction of melanogenesis. It classically manifests as hyperpigmented macules and patches distributed symmetrically on the face and neck.1–3 Known risk factors include ultraviolet (UV) radiation, hormonal influences, phototoxic medications, and genetic predisposition.1 Melasma can be an especially refractory form of hyperpigmentation causing psychosocial distress in many affected individuals.4
Some of the most prominent and successful treatments for hyperpigmentation are melanogenesis inhibitors that target tyrosinase, a enzyme crucial in the synthesis of melanin. Most commercially available skin-lightening cosmetics or agents that are tyrosinase inhibitors include hydroquinone, arbutin, ellagic acid, mulberry extract, kojic acid, and vitamin C.5 However, some tyrosinase inhibitors have been found to have limitations such as safety concerns, adverse reactions, poor skin penetration, and formulation challenges.6 Lumixyl peptide (decapeptide-12) was developed by dermatological researchers at Stanford University to specifically target tyrosinase without harming the melanocytes.7 It has been clinically shown to be safe and effective in improving facial melasma condition.8,9
In addition to targeting melanin production, combinatory therapies that also include molecules to stimulate cellular turn over and reduce skin inflammation have been shown to be effective in improving facial melasma condition.10,11 The Trifecting® Night Cream 1.0 (Envy Medical, Long Beach, California) is designed to be such a multimodality treatment. Besides the proprietary oligopeptide, Lumixyl®, it contains encapsulated retinol, which utilizes a submicron delivery system to ensure the penetration of retinol into the skin. Thus, its potency and efficacy is reserved until it fully reaches its cellular target. Furthermore, botanical extracts of bisabolol and bakuchiol are combined into a stable complex via the company’s patented PCX technology. These ingredients can effectively calm inflammation, neutralize free radicals, and function like retinol to boost cellular turnover. Combining the three key active ingredients, Trifecting® Night Cream 1.0 is expected to be an effective treatment for facial melasma condition.
This clinical trial was designed to evaluate the effects of long-term usage of Trifecting® Night Cream 1.0 in conjunction with a cleanser and sunscreen regimen in subjects with moderate to severe melasma, the presence of solar lentigines, and periocular lines and wrinkles.
Materials and Methods
Twenty-eight subjects (aged 36–65 years) with Fitzpatrick skin types II to IV who were in good general health with moderate to severe melasma (>25% coverage of melasma on the face), a presence of solar lentigines, and mild to moderate periorbital wrinkles and lines were enrolled into this study.
Subjects were provided with a facial regimen of two Envy Medical products (cleanser and sunscreen), with subjects in Cell 1 also receiving the Trifecting® Night Cream. Subjects were given detailed usage instructions (Table 1).

Institutional review board approval was obtained prior to commencement. This study was conducted according to ethical and regulatory principles of the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Prior to treatment, subjects provided informed consent and photoconsent. The study was conducted in Richardson, Texas, from August 2015 to March 2016.
Subjects were treated over a 24-week period with visits occurring at baseline and Weeks 4, 8, 16, and 24. After a four-week regression period, subjects were again evaluated at Week 28. Subjects arrived at the clinic having removed all makeup prior to the visit and the following assessments were performed at indicated time points.
Clinical efficacy. At baseline (except for Melasma Improvement Assessment and Investigator’s Global Improvement Assessment) and Weeks 8, 16, 24, and 28, an expert grader evaluated the face of each subject.
First, using a modified Griffiths 10-point scale, the following were obtained:12
- Fine Lines, periocular (0=none, 1–3=mild, 4–6=moderate, and 7–9=severe)
- Wrinkles, periocular (0=none, 1–3=mild, 4–6=moderate, and 7–9=severe)
- Solar lentigines, global face (Number: 0=none, 1–3=mild, 4–6=moderate, and 7–9=severe) (Average size: 0=none, 1–3=mild, 4–6=moderate and 7–9=severe)(Average intensity: 0=none, 1–3=mild, 4–6=moderate, and 7–9=severe)
- Additionally, the following were performed:
- Investigator’s Melasma Severity Assessment (0=none; 1=minimal/trace, covering 1%–10% area of the face; 2–3=mild, 11%–25%; 4–5=moderate, 26%–40%; 6–7=marked, 41%–50%; 8=severe, >50%)
- Melasma Pigmentation Intensity Assessment (0=none, 1=minimal, 2–3=mild, 4–5=moderate, 6–7=marked, 8=severe).
- Melasma Area and Severity Index (MASI)13 (pigment intensity [darkness], lesion size [area], and homogeneity evaluated for each of four areas of face; MASI calculated for entire face with 0=minimum, 48=maximum)
- Melasma Improvement Assessment (0=worse; 1=unchanged; 2=slight improvement, 1%–10%; 3=mild, 11%–25%; 4=moderate, 2%–50%; 5=marked, 51%–75%; 6=almost complete, 75-90%; 7=complete clearing, 91%–100%)
- Investigator’s Global Improvement Assessment (1=worse, 2=no improvement, 3=mildly improved, 4=moderately improved, 5=markedly improved)
Tolerability. At baseline and Weeks 4, 8, 16, 24, and 28, each subject was assessed by the investigator for objective evidence of erythema, dryness, scaling, and edema using a four-point scale (0=none to 3=severe). Subjects also assessed themselves for the irritation parameters of burning, stinging, and itching on a four-point scale (0=none to 3=severe).
Chroma Meter measurements. At baseline and Weeks 8, 16, 24, and 28, Chroma Meter CR-400 (Konica Minolta, Tokyo, Japan) measurements were taken to assess skin color on each of three pigmented spots selected by the investigator or designee (at least one spot was a solar lentigo).
Digital photography. At baseline and Weeks 8, 16, 24, and 28, digital photographs were taken using the VISIA CR2 (Canfield Imaging Systems, Fairfield, New Jersey). At each visit, subjects had three sets of full-face images taken (right side, left side, and center view) with standard lights 1 and 2, parallel polarized light, and cross-polarized light.
Image analysis. Digital photographs taken under cross-polarized lighting at baseline and at Weeks 8, 16, 24, and 28 were subjected to image analysis to assess skin tone evenness. The analysis was done using proprietary macros developed by Stephens & Associates using the Image Pro Plus version 7 software (Media Cybernetics, Inc., Rockville, Maryland).
Skin tone evenness analysis quantifies the variation of color intensity on defined areas of the face and reports skin tone evenness index, which is defined as the result of (standard deviation)/(average intensity) for the three color channels of red, green, and blue. A lower index value indicates a more even skin tone. The cheek area from the left view photos was selected for analysis.
Self-assessment questionnaires. At baseline and Weeks 8, 16, 24, and 28, subjects completed a self-assessment questionnaire regarding their experience with the facial regimen.
Statistical analysis. The per-protocol (PP) population was the primary population for all statistical analyses. The PP population included all subjects who received treatment and completed the study in general accordance with the protocol. The Wilcoxon signed-rank test was used for all clinical grading assessments and self-assessment questionnaires. The Wilcoxon rank-sum test was used for comparisons between treatments. A paired t-test was used for analyzing instrumentation data and image analysis data with a two-sample t-test used for comparisons between treatments. All differences were considered to be statistically significant at a p<0.05 level.
Results
The demographic data for the PP population are presented in Table 2. Twenty-five subjects completed the 28-week trial, with 14 subjects in Cell 1 and 11 subjects in Cell 2. Three subjects, all in Cell 2, discontinued (two were lost to follow-up and one requested withdrawal).

Efficacy assessed through clinical grading. Subjects in both groups showed improvements in facial conditions after products usage. Subjects in Cell 1 (active group) showed statistically significant improvement in all clinical grading parameters, including periorbital wrinkles, melasma, and solar lentigines, starting from Week 16 (Table 3). All of the subjects (100%) in Cell 1 showed improvement in periorbital fine lines starting at Week 16 and 100 percent of subjects showed improvement in MASI at Week 24. Subjects in Cell 2 (placebo group) showed statistically significant improvement in a subset of the parameters at Week 24 (Table 3).

Comparisons between cells, based on the mean change from baseline for clinical grading scores, indicated a statistically significant difference in favor of Cell 1 (active group) for periorbital fine lines, melasma pigmentation intensity assessment, number of solar lentigines, and Investigator’s Melasma Severity Assessment score (Figure 1).

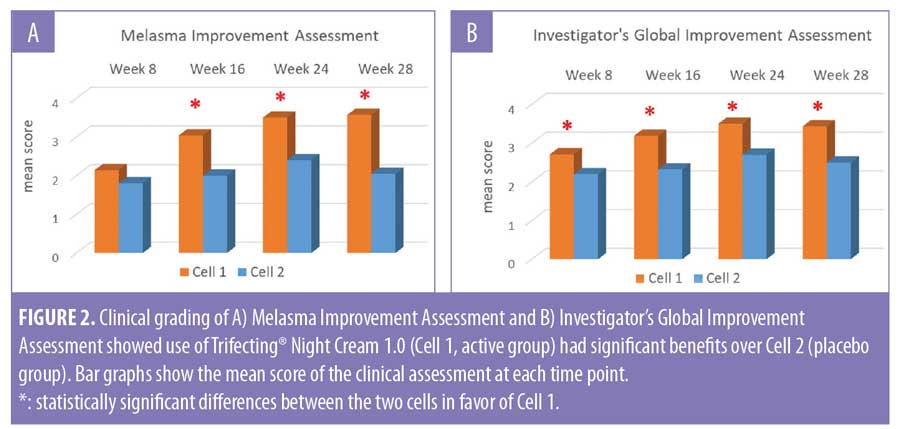
At the end of the 24-week treatment period, subjects in Cell 1 (active group) showed mild to moderate improvement in facial melasma condition with a mean score of 3.50 in the Melasma Improvement Assessment and 3.50 in the Investigator’s Global Improvement Assessment. These scores were significantly better than the improvement scores seen in Cell 2 (placebo group), which were 2.40 and 2.70, respectively (Figure 2). Importantly, the improvements achieved after 24 weeks of product usage were largely sustained during the four-week regression period (Figures 1 and 2).
Efficacy assessed through bioinstrumentation and image analysis. Three pigmented areas were selected for Chroma Meter measurement through the course of the study. The average b* value for all three sites showed statistically significant improvements for both groups (Figure 3), indicating a reduction in melanin concentration in the skin. The reduction in b* value was more significant in Cell 1 (active group) than in Cell 2 (placebo group). However, the difference between the two cells didn’t reach statistical significance.

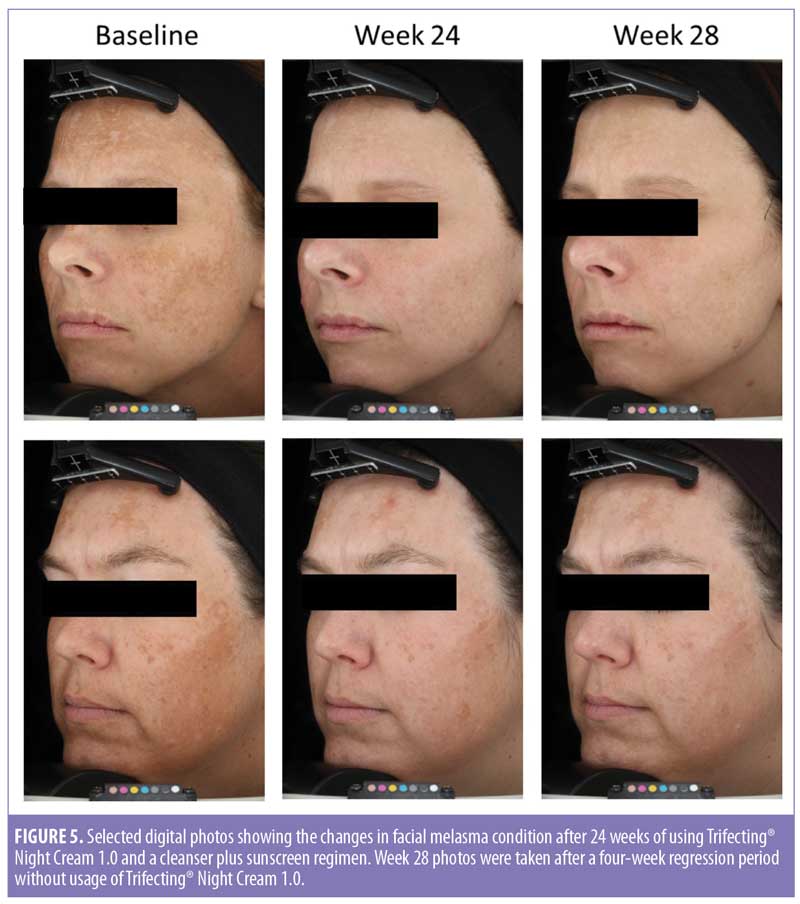
Image analysis for skin tone evenness was conducted upon trial completion. A statistically significant improvement in skin tone evenness index was achieved for both cells (Figure 4). Selected before and after treatment digital photos are shown in Figure 5.

Subjects satisfaction assessment through questionnaire. Subjects in both cells indicated satisfaction with the products used. Most people reported improvements in skin texture, skin tone evenness, skin brightness, radiance, and lines and wrinkles.
Safety and tolerability. The products were generally well tolerated by all subjects. However, there were six subjects in Cell 1 (active group) who reported irritation when using the test products during the trial. Symptoms included erythema, itching, and dryness. All were mild in nature.
Discussion
Melasma is prevalent in all ethnic groups. Its impact on appearance can cause significant psychosocial and emotional stress in many people.1–3 Due to its refractory nature and complex etiology, in recent years, researchers have resorted to combinatorial therapies to treat this condition.10,11 Envy Medical’s Trifecting® Night Cream 1.0 is a hydroquinone-free, triple-acting product designed to improve the appearance of facial melasma. The three key active ingredients, Lumixyl® peptide, encapsulated retinol, and botanical extracts of bisabolol and bakuchiol stabilized via PCX technology, are designed to inhibit tyrosinase, stimulate cellular turnover, and provide anti-inflammatory and antioxidant benefits. The combining of these active ingredients into one simple-to-use cosmetic cream would be ideal for people with a melasma condition.
A randomized, placebo-controlled, double-blind clinical study was conducted to demonstrate the benefits of long-term use of Trifecting® Night Cream 1.0 in conjunction with Envy Medical® cleanser and sunscreen in improving facial melasma condition. The efficacy was demonstrated through both clinical grading assessments and objective measurements (Chroma Meter and image analysis). Benefits were clearly seen as early as Week 8 with use of Trifecting® Night Cream 1.0. All subjects showed an improvement in periorbital fine lines starting at Week 16 and 100 percent of subjects showed an improvement in MASI at Week 24.
Limitations. Many of the assessment scores and values continued to improve and the difference between the two groups continued to widen over time. However, for some parameters, the difference between the two groups failed to reach the threshold of statistical significance at later time points (Figures 1b and 1d). A closer examination of the data indicated two issues. First, the sample size in this clinical trial was rather small. Second, subjects’ responses to Trifecting® Night Cream 1.0 showed some variation from person to person. Some showed drastic improvement (Figure 5), while a couple subjects showed rather small improvements. So, although the mean value for the population continued to improve, the standard deviation for the dataset became rather large, resulting in no statistical significance when comparisons were made. The latter point is of particular interest. As we enter the era of personalized medicine, personalized skin care products should also be designed and demanded. Further research is needed to better understand why people in this study responded to the triple-acting cream differently, what kind of melasma condition does not respond well to the treatment, and how to identify other pathways and mechanisms in combating melasma.
References
- Handel AC, Miot LD, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89(5):771–782.
- Sheth VM, Pandya AG. Melasma: a comprehensive update: part I. J Am Acad Dermatol. 2011;65(4):689–697.
- Ball Arefiev KL, Hantash BM. Advances in the treatment of melasma: a review of the recent literature. Dermatol Surg. 2012;38(7 Pt 1):971–984.
- Pawaskar MD, Parikh P, Markowski T, McMichael AJ, et al. Melasma and its impact on health-related quality of life in Hispanic women. J Dermatol Treat. 2007;18:5–9.
- Rodrigues M, Pandya AG. Melasma: clinical diagnosis and management options. Australas J Dermatol. 2015;56(3):151–163.
- Westerhof W, Kooyers TJ. Hydroquinone and its analogues in dermatology—a potential health risk. J Cosmet Dermatol. 2005;4:55–59.
- Abu Ubeid A, Zhao L, Wang Y, Hantash BM. Short-sequence oligopeptides with inhibitory activity against mushroom and human tyrosinase. J Invest Dermatol. 2009;129(9):2242–2249.
- Hantash BM, Jimenez F. A split-face, double-blind, randomized and placebo-controlled pilot evaluation of a novel oligopeptide for the treatment of recalcitrant melasma. J Drugs Dermatol. 2009 Aug;8(8):732–735.
- Hantash BM, Jimenez F. Treatment of mild to moderate facial melasma with the Lumixyl topical brightening system. J Drugs Dermatol. 2012;11(5):660–662.
- Hexsel D, Soirefmann M, Fernandes JD, Siega C. Objective assessment of erythema and pigmentation of melasma lesions and surrounding areas in long-term management regimens with triple combination. J Drugs Dermatol. 2014;13(4):444–448.
- Sheth VM1, Pandya AG. Melasma: a comprehensive update: part II. J Am Acad Dermatol. 2011;65(4):699–714.
- Griffiths CE, Wang TS, Hamilton TA et al. A photonumeric scale for the assessment of cutaneous photodamage. Arch Dermatol. 1992; 128:347–351.
- Kimbrough-Green CK, Griffiths CE, Finkel LJ, et al. Topical retinoic acid (tretinoin) for melasma in black patients. A vehicle-controlled clinical trial. Arch Dermatol. 1994;130:727–733.

