 J Clin Aesthet Dermatol. 2023;16(2):37–43.
J Clin Aesthet Dermatol. 2023;16(2):37–43.
by Rompu Roger Aruan, MD; Hernayati Hutabarat, MD; Arini Astasari Widodo, MD;
Maria Taurina Christabella Chandra Firdiyono, MD; Cecillia Wirawanty, MD; and Linda Fransiska, MD
All authors are with the Department of Dermatology and Venereology at Kristen Krida Wacana University in Jakarta, Indonesia.
ABSTRACT: Background. Crow’s feet is one of the signs of skin aging. Many studies regarding skin aging have been carried out in Caucasians, as for Asians, there are different genotypes and phenotypes. Some anti-aging treatments carry a slightly higher risk of side effects and irritation in Asian skin. Currently, the use of topical active peptides for anti-aging, Acetylhexapeptide-3 (AHP-3) and Palmitoyl pentapeptide-4 (PPP-4), has been widely developed. This study aimed to investigate the anti-aging effects of AHP-3 and PPP-4 on the Asian patient with crow’s feet.
Methods. This study was a double-blind randomized trial using 21 Indonesian female subjects aged 26 to 55 years for eight weeks and divided into three groups: AHP-3 cream, PPP-4 cream, and placebo. The cream was applied twice daily to the periorbital area. The three groups were assessed using Corneometer, Tewameter, Cutometer, digital photography and Crow’s Feet Grading Scale.
Results. Based on clinical photos and data, improvements were found in several subjects using AHP-3 and PPP-4. PPP-4 appeared to demonstrate better results when compared to AHP-3 based on data, clinical photos, and self-assessment questionnaire.
Conclusion. PPP-4 demonstrated better results when compared to AHP-3 and placebo. This initial study provides an opportunity for further study with a more adequate number of samples and duration.
Keywords: Acetylhexapeptide-3, Palmitoyl pentapeptide-4, crow’s feet
The increasing life expectancy, the ease of accessing information, the high interest and concern in caring for the skin have made skin care, especially anti-aging, increasingly popular among Asians. Unfortunately, research on anti-aging is dominated by Caucasian subjects who have different phenotypes and aging mechanisms from Asians.1,2,3 Nouveau-Richard et al’s4 study on Chinese and French Caucasian women showed that the earliest facial wrinkles appeared in the periorbital area (crow’s feet), forehead, followed by the area around the mouth (perioral).4
Along with the advancement of study in the field of anti-aging, many treatments can be done to prevent aging. However, studies show that some of the therapies have a slightly higher risk of side effects in people with colored skin type, including Asian races than in Caucasians, including erythema, keloids, and post-inflammatory hyperpigmentation.
A study by Blanes-Mira6 showed a 30-percent improvement in wrinkles in the periorbital area when a cream containing acetylhexapeptide-3 (AHP-3) was applied.7 Wang et al6 observed that wrinkles in the group using the AHP-3 cream showed 48.9-percent improvement compared to the group using the placebo cream (0%).6
In a double-blind study by Lintner8, palmitoyl pentapeptide-4 (PPP-4) cream (0.005%) applied to the area around the right eye twice daily for 28 days resulted in quantitative decrease in fold depth by 18 percent, fold thickness by 37 percent, and skin firness by 21 percent. Kaczvinsky et al9 showed PPP-4 can reduce the depth of wrinkles for four weeks in women with moderate to severe appearance of crow’s feet. Robinson et al10 studied Caucasian female subjects for 12 weeks, the subjects felt their skin was comfortable, fine lines and wrinkles were reduced, and irritation did not occur compared to the placebo group.
This study aims to investigate the antiaging effect of AHP-3 and PPP-4 on the crow’s feet in Asian subjects, specifically Indonesian subjects.
Methods
This study was a double-blind randomized trial that included 21 Indonesian women aged 26 to 55 years for eight weeks with crow’s feet. The inclusion criteria were Indonesian women aged 26 to 55 years, Fitzpatrick Skin Type III-V, healthy condition, crow’s feet in the periorbital area, willing to avoid sun exposure on the face, and willing to sign the informed consent. Potential participants were excluded from the study if they met any of the following criteria:
- Allergy to the test drug
- Systemic diseases that cause contraindications to participate in this study
- Skin diseases found in the tested skin area, such as psoriasis, vitiligo, and others
- Suspicious lesions or skin cancer in the area being tested
- Under dermatological treatment due to any condition on the area being tested
- Infections, burns, cuts, tattoos, scars, or excessive acne on the area being tested
- Pregnancy, breastfeeding, or trying to get pregnant three months before the study and or during the study
- Use of hormonal contraception
- Use of over-the-counter (OTC) topical products other than sunscreen, such as OTC acne treatment in the last one month
- Participated as a subject in a facial skin study within one month prior to the first visit to the study
- Performed facial skin resurfacing treatments (peeling, microdermabrasion, etc.) in the last six months
- History of active smoking
- Employees of examiners, market research companies, cosmetic/body and facial care manufacturers.
Drop out criteria was met if the participant was unable to follow the approved research protocol, was not present at the appointed examination, or was unable to use the given substance due to allergies or irritation.
All participants were divided into three treatment groups. Group I included seven subjects who had AHP-3 cream, Group II included seven subjects who had PPP-4 cream, and Group III had seven subjects who had placebo cream. The cream was applied to the periorbital area, especially the infraorbital and lateral periorbital of the right and left eyes twice daily as much as one finger tip unit (FTU) / 0.5 gram. The cream was applied with gentle movements and did not rub the area around the eyes.
The three groups were assessed using corneometer, tewameter, cutometer, digital photography, and Crow’s Feet Grading Scale. The Crow’s Feet Grading Scale was applied to two separate evaluations of crow’s feet, such as at rest (static) and with expression (dynamic) and was performed on a 5-point ordinal scale (0=no wrinkles, 1=very fine wrinkles, 2=fine wrinkles, 3=moderate wrinkles, 4=severe wrinkles).15
Measurements were performed at baseline, Week 0 (W0), Week 4 (W4), Week 6 (W6), and Week 8 (W8) and were carried out after the subject has cleansed their face, allowed the skin to rest for at least 10 minutes, and subject self-assessment had been completed. Subject assessed product efficacy rating (1=very disagree, 2=disagree, 3=neutral, 4=agree, 5=very agree) and cosmetic qualities rating (1=very poor, 2=poor, 3=average, 4=good, 5=very good).
Corneometer, tewameter, cutometer. The corneometer is an instrument used to evaluate the hydrating effect of cosmetic treatments on skin moisture. A corneometer can measure to a depth of 10-20µm in the stratum corneum in the epidermis. Tewameter is a tool used to evaluate transepidermal water loss (TEWL). Cutometer is a tool used to evaluate the skin’s viscoelasticity, which is the capacity of the skin to return to its original shape after deformation.11,13 The cutometer parameters include R0, R5, and R7 (R0=the final distension of the first curve, R5=the net elasticity, R7=the ratio of elastic recovery to the total deformation).13
Statistical analysis. From the evaluation results, data processing and statistical analysis were carried out using the Anova Repeated Measurement test or Kendall test. Crow’s feet grading scale was reported with descriptive statistics (mean) and change from baseline. Subject self-assessments and cosmetic qualities were reported as frequency (n,%) for each response and were analyzed using Chi Square test. A P-value <0.05 was considered significant.
Results
Of the 36 subjects who registered for the study, there were 21 subjects who met the inclusion criteria and were included. Baseline demographic and clinical characteristics are shown in Table 1.

Based on the Anova Repeated Measurement or Kendall test, the p value of corneometer, tewameter, and cutometer result was not significant (p>0.05) both right (Table 2) and left eye crow’s feet (Table 3). The insignificant p-value could be caused by the small number of samples, the short duration of the study, and the difference in tool sensitivity. Right (Figure 1) and left eye crow’s feet (Figure 2) corneometer, tewameter, and cutometer assessment result chart between W0 and W8 were reported to facilitate data analysis.




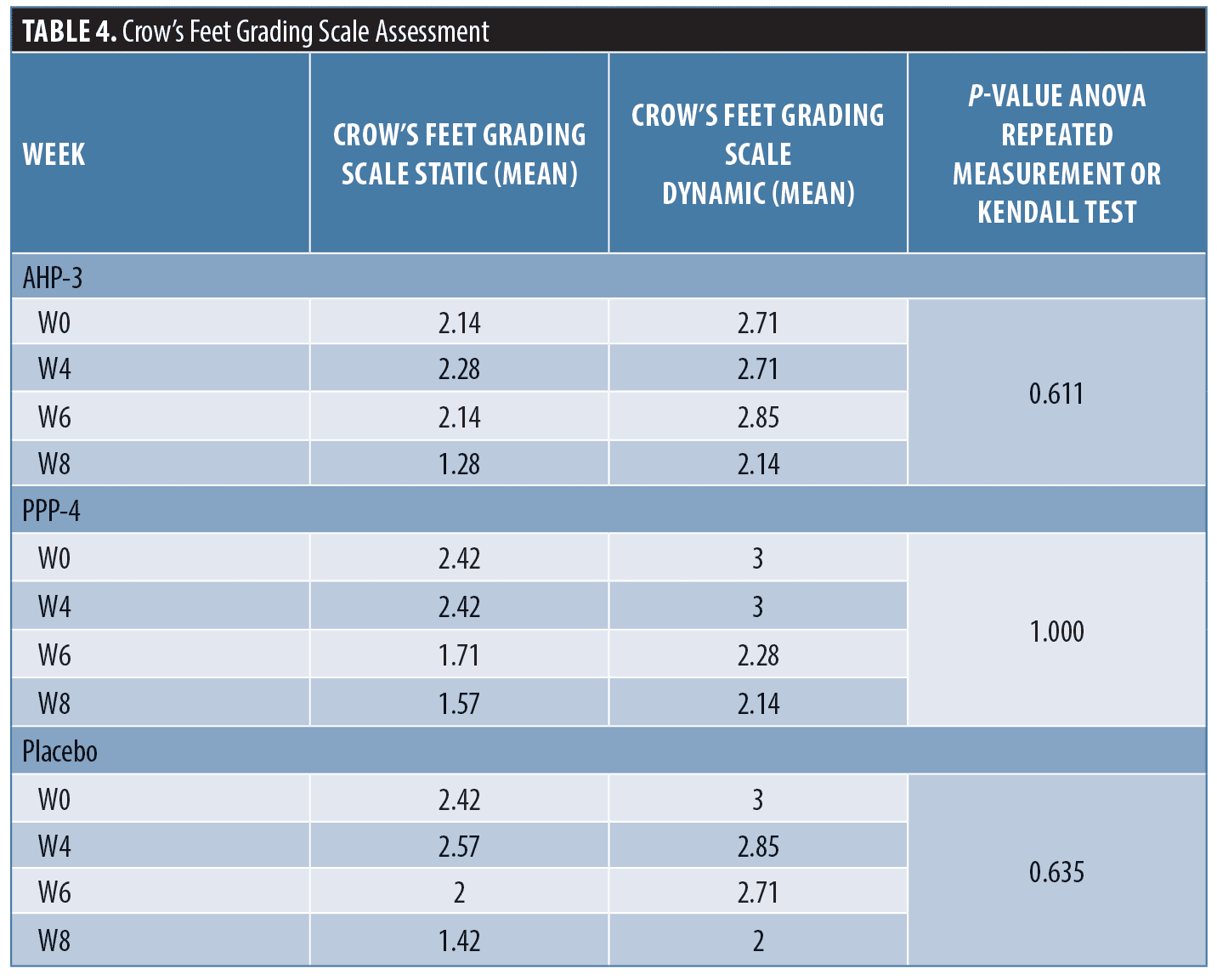
Crow’s feet grading scale static and dynamic mean were calculated (Table 4). The mean crow’s feet grading scale scores decreased at Week 8 compared to baseline. The crow’s feet grading scale on the administration AHP-3 decreased 0.86 points (static) and 0.57 points (dynamic) at Week 8 while on the administration PPP-4 decreased 0.86 points (static and dynamic) at Week 8 (Figure 3).

The researchers made their assessment based on clinical photos and data that had improved on the corneometer, tewameter, and cutometer at W0 and W8.
In the AHP-3 group, Subject 3 showed an improvement. In the static photo/without expression of W0, the fine lines were seen (blue and green arrows) and the infraorbital appeared darker (grey arrow). Meanwhile, at W8, there was an improvement. The fine lines were reduced (red and purple arrows) and the infraorbital appeared brighter (orange arrows).
In the photo given the facial expression, a significant change in the crow’s feet was seen. At W0, the crow’s feet were very clearly visible (blue, green, brown, and yellow arrows) and the infraorbital appeared darker (grey arrow). Meanwhile, at W8, there was an improvement. The crow’s feet were reduced (red, purple, pink, black arrows) and the infraorbital appeared brighter (orange arrow) (Figure 4). These clinical photos were in accordance with the results of the corneometer and cutometer of Subject 3 (Figure 5).


In the PPP-4 group, Subject 15 and 36 showed an improvement. In the static photo at W0, the crow’s feet of the Subject 15 were not clearly visible, fine lines were seen (blue and green arrows). Meanwhile, at Week 8, there was an improvement. The fine lines were reduced (red and purple arrows).In the photo facial expressions of Subject 15, significant changes in the crow’s feet were seen. At W0, crow’s feet were very clearly visible (blue, green, brown, yellow arrows). Meanwhile, at W8, the crow’s feet were reduced (red, purple, pink, black arrows) (Figure 6). In the static photo of Subject 36 at W0, the crow’s feet were not clearly visible, but fine lines were seen (blue and green arrows). While at W8, the fine lines were reduced (red and purple arrows). In the photo facial expressions, at W0, the crow’s feet were very clearly visible (blue and brown arrows). Meanwhile, at W8, the crow’s feet were reduced (red, pink, and black arrows) (Figure 7). These clinical photos were in accordance with the results of the corneometer, tewameter and cutometer of Subject 15 and 36 (Figures 8 and 9).




In the placebo group, Subject 26 showed an improvement. In the static photo at W0, the crow’s feet were not clearly visible. However, the infraorbital appeared darker (grey arrow). Meanwhile at W8, the infraorbital area appeared brighter (orange arrow). In the photo facial expressions at W0, the crow’s feet were very clearly visible (blue, green, brown arrows) and the infraorbital appeared darker (grey arrow). Meanwhile, at W8, the crow’s feet were reduced (red, purple, pink arrows) and the infraorbital area looked brighter (orange arrow) (Figure 10). These clinical photos were in accordance with the results of the corneometer, tewameter and cutometer of Subject 26 (Figure 11).


Subject self-assessment. No serious adverse events occurred. One subject using AHP-3 cream experienced itching (14%), two had stinging (29%), one had burning (14%), one had oily in the periorbital (14%) and one had dry skin (14%). Two subjects using placebo cream experienced itching (29%), two had burning (29%), one had oily in the periorbital (14%), one had sticky skin (14%), and one had dry skin (14%). Meanwhile, subjects using PPP-4 cream had no complaints.
Cosmetic quality assessments. Table 5 shows the scores for each assessment. Based on these data, the p value of the subjective assessment of the research subjects on the three creams was not significant ( >0.05). However, based on the questionnaire, it is evident that the subjects rated the quality of PPP-4 cream better than AHP-3 cream and placebo: at Week 8, 5/21 rated very good, 2/21 rated good (Table 5).

Discussion
Topical peptides for cosmetic use consist of four major groups, namely signal peptides, carrier peptides, neurotransmitter-inhibitory peptides, and enzyme inhibitor peptides. Synthetic/artificial peptides have amino acid chains that can be modified into various amino acid chains that have special properties, such as increasing molecular stability so that peptides are not easily damaged.5,12
The way the peptides work from each group is different. In signaling peptides, peptides stimulate the production of extracellular matrix so that more skin supporting substances (collagen, elastin, etc.) are produced and the skin looks firmer. Carrier peptides carry essential elements needed for wound healing and enzymatic processes into skin cells. Neurotransmitter-inhibitory peptides can relax muscles thereby reducing the appearance of wrinkles and fine lines. Peptide inhibitor enzymes work directly or indirectly by inhibiting the action of certain enzymes.5
AHP-3 is an analogue of synaptosomal-associated protein 25 (SNAP-25) that compete for the same position on the SNARE complex. AHP-3 then releases the formation of the SNARE complex without damaging the proteins and other substances present in the SNARE complex. In addition, AHP-3 also inhibits catecholamine secretion.5
AHP-3 has the amino acid chain sequence Acetyl-Glu-Glu-Met-Gln-Arg-Arg-NH2 and works by inhibiting the release of neurotransmitters thereby inhibiting muscle contraction. The effect can reduce wrinkles and fine lines and improve skin firmness.5,12
AHP-3 works by reducing muscle contractions of facial expressions.6 This is what is currently being sought by consumers who want to avoid invasive procedure.
PPP-4 has the amino acid chain sequence Pal-Lys-Thr-Thr-Lys-Ser-OH or pal-KTTKS-OH. PPP-4, including the type of signaling peptide, is a very specific peptide that stimulate the production of elastin, fibronectin, glucosaminoglycans and collagen (especially Types I, III and IV) and accelerate wound healing.5
This study showed improvements in the crow’s feet of patients taking AHP-3 and PPP-4 when compared to placebo. Statistical results showed that there was no significant difference between these two active substances. Limitations of this study include insufficient number of samples and the short duration of the study. Although the duration of the study had been planned according to the previous studies, the results were still not statistically adequate. The level of satisfaction in all groups was good and the positive benefits of using the cream were found based on a questionnaire survey. PPP-4 showed better satisfaction rate than AHP-3.
Conclusion
Improvements in crows feet were measured in several subjects using AHP-3 and PPP-4. PPP-4 provides better results when compared to AHP-3 and placebo. The superiority of PPP-4 requires study with a better design. This initial study provides an opportunity for further study with a more adequate number of samples and duration.
Acknowledgments
This study was supported by Kristen Krida Wacana University.
References
- de Araújo, R Lôbo, M, Trindade K et al. Fibroblast growth factors: Acontrolling mechanism of skin aging. Skin Pharmacol Physiol. 2019;32,275–282.
- Venkatesh, S, Maymone, MBC, Vashi, NA. Aging in skin of color. Clin Dermatol. 2019;37, 351–357.
- Knaggs, H. Skin aging in the asian population. In Skin Aging Handbook: An Integrated Approach to Biochemistry and Product Development;Dayan, N.; Publisher: New York, USA, 2008;Chapter 9, pp. 177–201.
- Nouveau-Richard S, Yang Z, Mac-Mary S, et al. Skin aging: A comparison between Chinese and European populations: A pilot study. J Dermatol Sci. 2005, 40, 187–193.
- Schagen, SK. Topical peptide treatments with effective anti-aging results. Cosmetics. 2017, 4,16.
- Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of argireline, a synthetic hexapeptide, in chinese subjects: A randomized, placebo-controlled study. Am J Clin Dermatol. 2013,14, 147– 153.
- Blanes-Mira C, Clemente J, Jodas G, et al A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002, 24, 303–310.
- Lintner, 2003. Cosmetic or Dermopharmaceutical Use of Peptides for Healing, Hydrating and Improving Skin Appearance during Natural or Induced Ageing (Heliodermia, Pollution). Available online: https://patents.google.com/patent/US6620419B1/en (accessed on 29th April 2022).
- Kaczvinsky JR, Griffiths CEM, Schnicker, MS, et al. Efficacy of anti-aging products for periorbital wrinkles as measured by 3-D imaging. J Cosmet Dermatol. 2009,8, 228–233.
- Robinson LR, Fitzgerald NC, Doughty DG, et al. Topical palmitoyl pentapeptide provides improvement in photoaged human facial skin. Int J Cosmet Sci. 2005,27, 155–160.
- Constantin MM, Bucur S, et al. Measurement of skin viscoelasticity: A non-invasive approach in allergic contact dermatitis.
Experimental and Therapeutic Medicine. 2020,20,184. - Gorouhi F and Maibach HI. Role of topical peptides in preventing or treating aged skin. Int J Cosmet Sci. 2009,31, 327–345.
- Ohshima H. Use of cutometer area parameters in evaluating age-related changes in the skin elasticity of the cheek. Skin Research and Technology. 2012,1–5.
- Khan A. Visio scan VC98, corneometer MPA 5 and tewameter MPA 5. African J Pharm Pharmacol. 2012, 6, 225–227.
- Carruthers A, Carruthers J, Hardas B, et al. A validated grading scale for crow’s feet. Dermatologic Surg. 2008, 34, 173–178.

