 by Sharleen St. Surin-Lord, MD; Todd E. Schlesinger, MD, FAAD; and Eric Guenin, PharmD, PhD, MPH
by Sharleen St. Surin-Lord, MD; Todd E. Schlesinger, MD, FAAD; and Eric Guenin, PharmD, PhD, MPH
Dr. St. Surin-Lord is with Visage Dermatology and Aesthetics Center in Largo, Maryland. Dr. Schlesinger is with the Clinical Research Center of the Carolinas in Charleston, South Carolina. Dr. Guenin is with Ortho Dermatologics in Bridgewater, New Jersey.
FUNDING: Ortho Dermatologics funded the preparation of this manuscript.
DISCLOSURES: Dr. St. Surin-Lord is a paid consultant for Bausch Health. Dr. Guenin is and employee of Ortho Dermatologics. Dr. Schlesinger has no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. The efficacy of tretinoin is well documented in adolescent acne, with limited data available in preadolescents. A novel tretinoin 0.05% lotion formulation has been shown to be effective and well tolerated in moderate-to-severe acne.
Objective. We sought to evaluate the safety and efficacy of tretinoin 0.05% lotion in preadolescent (13 years or younger) and adolescent (14–17 years) subjects with acne.
Methods. This study involved the post-hoc analysis of two multicenter, randomized, double-blind, vehicle-controlled Phase III studies. Preadolescent (n=154) and adolescent (n=575) subjects were randomized (1:1) to tretinoin 0.05% lotion or vehicle used once daily for 12 weeks. Efficacy assessments included lesion count reductions, treatment success (at least a two-grade reduction in the Evaluator’s Global Severity Score and clear/almost clear). Safety, adverse events (AEs), and cutaneous tolerability were evaluated.
Results. At Week 12, mean percent reductions in inflammatory and noninflammatory lesion counts were 49.5% and 44.0% in preadolescents and 50.5% and 41.2% in adolescents, compared to 31.4%, 18.8%, 35.9%, and 23.8% for the vehicle, respectively (all p less than or equal to 0.001). Treatment success was achieved by 23.7% (preadolescent) and 17.5% (adolescent) of subjects by Week 12, compared to 7.2% (p=0.009) and 8.8% (P=0.004) with the vehicle. The majority of AEs were mild and transient; the most frequent occurrences were application site pain and dryness in 5.6% and 2.8% of preadolescents and 3.2% and 3.6% of adolescents. Local cutaneous safety and tolerability assessments were generally mild-to-moderate, with slight transient increases in mean scores at Week 4.
Conclusions. Tretinoin 0.05% lotion was significantly more effective than the vehicle in achieving treatment success and reducing inflammatory and noninflammatory lesions in preadolescent and adolescent acne. It was well tolerated, and all treatment-related AEs were mild or moderate.
KEYWORDS: Acne vulgaris, adolescent, moderate, preadolescent, severe, tretinoin, topical
J Clin Aesthet Dermatol. 2019;12(9):E57–E61
Acne vulgaris (acne) is a common, chronic skin disease that is principally an adolescent disorder. However, with an increasingly younger age of onset, preadolescent acne has become an important focus, especially as it might be predictive of future disease severity.1 The majority of clinical trials for acne treatments are conducted in subjects 12 years of age or older; consequently, few studies exist that can be used to compare results in a preadolescent and adolescent population. Large epidemiology studies have shown that acne is more prevalent in girls at younger age ranges, with an increasing prevalence in boys as they reach puberty2,3; male subjects also tend to have more severe acne.4–6 Longitudinal studies in American schoolchildren showed that prevalence and severity increased with pubertal maturation with comedonal acne predominant in preteens, with increasing inflammatory acne appearing during the teenage years.7,8
Recently, clinical efficacy and safety data on a novel tretinoin 0.05% lotion were published.9 Subjects as young as nine years old were included. Overall, tretinoin 0.05% lotion was significantly more effective than the vehicle in treating moderate or severe acne, with a highly favorable safety and tolerability profile. The incidence rates of erythema, dryness, and skin burning were lower than those previously reported with other formulations of tretinoin.10 Here, we present a post-hoc analysis of the two Phase III studies to evaluate the differences in efficacy and safety in preadolescent and adolescent subjects.
Methods
Study design. A post-hoc analysis of two multicenter, randomized, double-blind, vehicle-controlled, parallel-group clinical studies was conducted, with a focus on preadolescent and adolescent subjects with moderate or severe acne. Protocols for the pivotal trials received approval before patient enrollment from the appropriate institutional review boards and were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and in compliance with local regulatory requirements. All subjects were informed of the study details and provided written consent.
Study population. Eligible subjects included male and female patients of any race and ethnicity, aged 9 to 17 years old, who presented with 20 to 40 inflammatory lesions (i.e., papules, pustules, and nodules), 20 to 100 noninflammatory lesions (i.e., open and closed comedones), and two nodules or less. A washout period of up to one month was required for patients who had used prescription and/or over-the-counter acne treatments; a six-month washout period was required for those who had used systemic retinoids. More specifically, the following mandatory washout periods and restrictions applied to these topical and systemic treatments: topical astringents and abrasives (1 week); topical antiacne products, including soaps containing antimicrobials, and known comedogenic products (2 weeks); topical retinoids, retinol, and systemic acne treatments, such as hormonal or antibiotic treatments (4 weeks); and systemic retinoids (6 months).
Subjects with an Evaluator Global Severity Score (EGSS) score of three (moderate) or four (severe) points were enrolled and randomized (1:1) to tretinoin 0.05% lotion or vehicle applied once-daily for 12 weeks.
Efficacy evaluation. Efficacy evaluations comprised inflammatory and noninflammatory lesion counts and an EGSS assessment at screening, baseline, and subsequent study visits at Weeks 4, 8, and 12. Efficacy endpoints included mean percent change from baseline to Week 12 in inflammatory and noninflammatory lesion counts and the proportion of subjects achieving at least a two-grade reduction from baseline EGSS and “clear” or “almost clear” at that same visit.
Additional assessments included a patient satisfaction score (PSS) and a validated, acne-specific quality of life (Acne-QoL) questionnaire (Merck & Co., Inc. Kenilworth, New Jersey). At baseline, subjects were asked to rate their satisfaction with prior acne therapy with a PSS of 1 to 10 points, where 10 points was the most satisfied and five points or greater was considered as being “satisfied”. At Week 12, the subjects were asked to rate their level of satisfaction with the study treatment. Acne-QoL was evaluated using a 19-item, patient-reported outcome measure evaluating the impact of facial acne. The questionnaire consisted of four domains: self-perception, role-emotional, role-social, and acne symptoms. Each question was evaluated using a seven-point scale, where 1=“none” or “not at all” and 7=“extremely” or “extensive;” answers were grouped into the appropriate domains. Higher Acne-QoL domain scores indicated better acne-related QoL.
Safety evaluation. Cutaneous safety (erythema and scaling) and tolerability (itching, burning, and stinging) were evaluated on a scale from 0 to 3 points, where 0=none and 3=severe. Adverse events (AEs) were evaluated throughout, and their severity and relationship to the study medication were assessed.
Statistical analysis. The intent-to-treat (ITT) population included all patients randomized and provided with the study drug and vehicle. The safety population included all randomized patients who were presumed to have used the study medication or vehicle at least once and who underwent at least one postbaseline evaluation. The primary method of handling missing efficacy data in the ITT analysis set was based on estimation using the Markov Chain Monte Carlo multiple imputation method. No imputations were made for missing safety data.
Reductions in lesion counts were presented as least square (LS) means and treatment p-values from an analysis of covariance with values adjusted for multiple imputations. The significance of EGSS reductions was obtained from logistic regression, using Firth’s Penalized Likelihood, with factors of treatment group and analysis center. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Cary, North Carolina) or later. Statistical significance was based on two-tailed tests of the null hypothesis resulting in p-values of 0.05 or less.
All AEs occurring during the studies were recorded and classified on the basis of medical dictionary for drug regulatory activities terminology (MedDRA) for the safety population. Treatment group comparisons were made by tabulating the frequency of patients with one or more AEs during the study.
Results
Baseline characteristics. A total of 729 subjects with acne were included in the post-hoc analysis (154 preadolescents and 575 adolescents; Figure 1). Overall, 637 subjects (87.4%) completed the two studies, including 313 patients (85.1%) on tretinoin 0.05% lotion and 324 (89.8%) on vehicle. Overall, the most common reasons for study discontinuation were “lost to follow up” (n=42; 6.6%) or “subject request” (n=23; 3.6%). Four subjects treated with tretinoin 0.05% lotion discontinued due to AEs.

There was a predominance of female subjects (59.1%) in the preadolescent subgroup (Table 1) and male subjects (63.3%) in the adolescent group, respectively. Mean age (standard deviation [SD]) in the preadolescent group was 12.4 (0.94) years (range: 9–13 years) and 15.6 (1.08) in the adolescent group (range: 14–17 years). Subjects were predominantly Caucasian (n=115; 74.4% and n=458; 79.7%) in both groups.

There were no noticeable differences between the treatment groups with regard to baseline lesion count or EGSS. At baseline, the mean numbers (SDs) of inflammatory and noninflammatory lesions were 26.7 (6.04) and 50.3 (21.90) in the preadolescent group and 27.0 (5.83) and 46.5 (20.17) in the adolescent group. At baseline, 94.2 percent and 88.7 percent of subjects, respectively, had moderate acne (EGSS of 3 points).
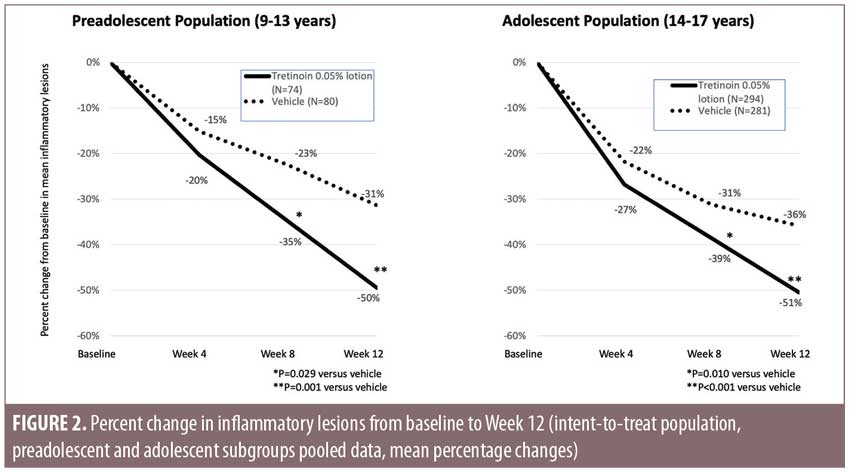
Efficacy. Tretinoin 0.05% lotion resulted in statistically significant reductions in both inflammatory and noninflammatory lesion counts (Figures 2 and 3). At Week 12, mean percentage changes (LS mean) from baseline in inflammatory lesion counts for the preadolescent and adolescent subgroups were 49.5 and 50.5 percent, respectively, compared to 31.4 and 35.9 percent for vehicle (both p less than or equal to 0.001). Noninflammatory lesion counts were reduced by 44.0 and 41.2 percent, respectively, compared to 18.8 and 23.8 percent for vehicle (both p less than or equal to 0.001).

Treatment success was defined as at least a two-grade improvement in global severity according to EGSS and being graded as “clear” or “almost clear.” By Week 12, 23.7 and 17.5 percent of the preadolescent and adolescent populations were treatment successes following treatment with tretinoin 0.05% lotion as compared with 7.2% and 8.8% on vehicle (p=0.009 and p=0.004; Figure 4).

Overall patient satisfaction with treatment was significantly greater with tretinoin 0.05% lotion than vehicle by Week 12 in both populations. At baseline, scores of satisfaction with prior acne treatment for those subjects who went on to receive tretinoin 0.05% lotion were 5.2 in preadolescents and 4.8 in adolescents. At Week 12, the score for satisfaction with tretinoin 0.05% lotion was was 7.0 in both groups (p=0.008 and p=0.002, respectively). There were no statistically significant differences in the improvement between treatment groups based on the mean Acne-QoL assessments for each domain.
Safety. There were more treatment-emergent AEs in the preadolescent group (Table 2). Most AEs were of a mild or moderate severity. Four adolescent subjects reported severe AEs in the tretinoin 0.05% lotion group. No severe AEs were considered treatment-related. Five subjects (1 preadolescent and 4 adolescents) treated with tretinoin 0.05% lotion discontinued due to treatment-emergent AEs. Treatment-related AEs with tretinoin 0.05% lotion were uncommon, less than those in the overall study population, and included application site pain (5.6% and 3.2% of preadolescents and adolescents), dryness (2.8% and 3.6% of preadolescents and adolescents), and erythema (1.8% in the adolescent group).

Cutaneous safety and tolerability. Erythema and scaling were recorded by the investigator, while itching, burning. and stinging severity scores were obtained from the patients. In all cases, tretinoin 0.05% lotion was well-tolerated. In the preadolescent population, there were slight, transient increases in mean scores for scaling and burning at Week 4, but all scores remained as 0.5 points or less where one point indicated mild (Figure 5A). There were slight, transient increases in scaling, burning, and stinging at Week 4 in the adolescent population, with no mean scores of less than 0.6.

Discussion
Acne is predominantly an adolescent disease, although it is increasingly more common in preadolescents. Most clinical studies have excluded children under the age of 12 years, so a comparison between efficacy and safety in both populations has been limited. In two Phase III studies with tretinoin 0.05% lotion, subjects aged nine years and older were included and, as a result, this post-hoc analysis was able to present comparative data. Although these were clinical trials, some of the baseline demographics and characteristics match those we see in clinical practice. For example, there was a higher proportion of female subjects in the preadolescent population, while there were more male subjects in the adolescent population, where there were also more subjects with severe acne.
Overall, within the two groups, lesion count reductions were similar and significantly greater than with vehicle. Comedonal acne tends to predominate in the preadolescent years, with increasing inflammatory acne appearing during adolescence. Tretinoin 0.05% lotion was more effective in reducing comedonal lesion counts in preadolescents than adolescents (44.0% vs. 41.2% reduction at Week 12), and the delta (active minus vehicle) was much more remarkable in preadolescents (25.2% vs. 17.4%). Tretinoin 0.05% lotion was equally effective in reducing inflammatory lesions in both the preadolescent and adolescent groups (49.5% and 50.5% lesion count reduction, respectively). Treatment success in the preadolescent subjects appeared to be better, although this finding could have been influenced by the fewer severe acne cases observed (2.7% vs. 10.5% in the adolescent group).
Tolerability was good in both groups. The incidence of treatment-related AEs was lower in the adolescent population (7.8% vs. 9.7%); in both groups, application site pain (5.6% and 3.2% in the preadolescent and adolescent populations, respectively) and dryness (2.8% and 3.6%, respectively) were the most common. The slight, transient increases in scaling and burning seen in both groups were anticipated. There were also small transient increases in mean scores for erythema, itching, and stinging in the adolescent subjects. Nevertheless, the incidence of treatment-emergent AEs was similar to that reported in the overall study population (8.1% overall and 3.1% and 3.7% for application site pain and dryness, respectively) and lower than in studies including other tretinoin formulations. For example, a comparative study with tretinoin 0.05% gel and tretinoin 0.1% microspheres reported incidences of skin dryness, burning, and erythema of 30, 15, and 18 percent versus 16, 8 and 7 percent for the two formulations of tretinoin.11
Conclusions
Tretinoin 0.05% lotion was significantly more effective than vehicle in achieving treatment success and lesion count reduction in preadolescent and adolescent acne, with similar results observed in both groups. It was particularly effective in reducing comedonal lesions in preadolescents and effective in reducing inflammatory lesions in both groups. Further, it was well tolerated, and all treatment-related AEs were mild or moderate and less frequent than reported with other tretinoin formulations.
Acknowledgments
The authors acknowledge Brian Bulley, MSc, of Konic Limited for medical writing support.
References
- Lucky AW, Biro FM, Sumbarti LA, et al. Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pedatr. 1997;130(1):30–39.
- Aksu AEK, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26(12):1503–1509.
- Kilkenny M, Merlin K, Plunkett A, et al. The prevalence of common skin conditions in Australian school students: 3, Acne vulgaris. Br J Dermatol. 1998; 139(5):840–845.
- Uslu G, Sendur N, Uslu M, et al. Acne: prevalence, perceptions and effects on psychological health among adolescents in Aydin, Turkey. J Eur Acad Dermatol Venereol. 2008;22(4):462–469.
- Aktan S, Ozmen E, Sanli B. Anxiety, depression, and nature of acne vulgaris in adolescents. Int J Dermatol. 2000;39(5):354–357.
- Shahzad N, Nasir J, Ikram U, et al. Frequency and psychosocial impact of acne on university and college students. J Coll Physicians Surg Pak. 2011;21(7):442–443.
- Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in early adolescent boys. Correlations with pubertal maturation and age. Arch Dermatol. 1991;127(2):210–216.
- Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. An early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130(3):308–314.
- Tyring SK, Kircik LH, Pariser DM, et al. Novel Tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: Assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17(10):1084–1091.
- Harper JC, Roberts WE, Zeichner JA, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of safety and tolerability in subgroups. J Dermatolog Treat. 2019:1–8. [Epub ahead of print].
- Webster G, Cargill I, Quiring J, et al. A combined analysis of 2 randomized clinical studies of tretinoin gel 0.05% for the treatment of acne. Cutis 2009;83(3):146–154.

