 J Clin Aesthet Dermatol. 2022;15(1):42–47.
J Clin Aesthet Dermatol. 2022;15(1):42–47.
by Lawrence S. Moy, MD; Jacob M. Hands, BA; and Paul K. Shitabata, MD
Dr. Moy and Mr. Hands are with the South Bay Institute of Clinical Research in Hermosa Beach, California. Dr. Shitabata is with Harbor-UCLA Dermatology and David Geffen School of Medicine at UCLA.
ABSTRACT: Background. There exists significant heterogeneity in the presentation of “common” skin cancers such as cutaneous melanoma (CM), cutaneous squamous cell carcinoma (cSCC) and basal cell carcinoma (BCC). Meaningful differences are often observed among the trio concerning age, sex, site at presentation and laterality.
Objective. In this paper, we endeavor to elucidate such heterogeneity, reaffirm burgeoning trends in skin cancer incidence, and offer new insights in the presentation of common skin cancers.
Results. While agreement with current consensus was achieved with regard to various aspects of sex, age, and site-specific findings, several novel results emerged: (1) the percentage of subjects presenting with CM was demonstrably higher than population averages would estimate; (2) melanoma exhibited a pronounced right-side bias; (3) cSCC was not head and neck preferring as other reviews have documented (4) cSCC exhibited greater female bias.
Conclusion. In this study, we documented insights from 663 cases (397 unique subjects) across a range of factors including age, laterality, site of presentation, and sex specific differences in incidence. The results of our analysis generally accord well with previous findings, replicating several of the most prominent results.
There exists significant heterogeneity in the topical manifestation of “common” skin cancers such as basal cell carcinoma (BCC), cutaneous squamous cell carcinoma (cSCC) and cutaneous melanoma (CM). These include differences in age, laterality, site-specific presentation and sex incidence. Yet, there have been relatively few complete cohort studies that have examined each factor collectively. We relay insights from a three-year observational cohort in Manhattan Beach, California, presenting to a dermatology clinic for routine evaluation. In total, 663 unique cases (397 subjects) were identified and diagnosed with either BCC, cSCC, or CM via shave, punch, or excisional biopsy from 2017 to 2019. We report insights from the cohort with respect to age at diagnosis, laterality, site of presentation, and sex differences in incidence. We offer explanation for our observed results and compare findings against novel trends and accepted beliefs with respect to skin cancer incidence across a range of factors.
Cohort Demographics
The present study consists of 663 unique cases (397 subjects) diagnosed with a skin-related malignancy from the years 2017 to 2019. All subjects identified were residents of Los Angeles, California, at the time of presentation without a prior change of primary residence outside of Los Angeles in the past 10 years.
Inclusion criteria were defined as those subjects presenting with either basal cell carcinoma (BCC), cutaneous squamous cell carcinoma (cSCC) or cutaneous melanoma (CM) from 2017 to 2019. Subjects presenting with types of skin cancer other than those delineated above were excluded. Subjects with multiple malignancies during the study period were recorded. Subjects were identified during primary evaluation at a clinic in Manhattan Beach, California, screened, and received a final diagnosis of either BCC, cSCC, CM or a combination confirmed during follow-up dermatopathologic evaluation. Subjects ranged in age from 21 to 97 years, with a median age of 68 (+ 12.4). Sex breakdown for participants was as follows: male (67.1%) & female (32.9%). Though subjects were predominantly male, both groups were roughly age matched (median age = 66.1 (+ 12.2) v. 67.6 (+ 12.6)) for males and females, respectively.
Methodology & Statistics
Three hundred and ninety-seven subjects were screened from 2017 to 2019 yielding 663 unique cases of BCC, cSCC and CM. Subjects were seen on a voluntary basis and evaluated by a board-certified dermatologist and received follow-up diagnosis from a board certified dermatopathologist. Biopsy was performed via shave, punch, and excision. Age at diagnosis, laterality of skin malignancy, sites of presentation and subject sex were recorded.
Subject sex was categorized as “Male” or “Female” (denoted as a binary variable with Male is “1” and Female “0”). “Malignancy” was recorded as a binary observation where a subject with a positive diagnosis is “1” and a subject with a negative diagnosis is “0” across each of the three categories representing BCC, cSCC or CM. “Laterality” was defined as the appearance of skin malignancy on the left or right side of the body, site independent. Laterality was defined as a binary variable where left sided appearance was “1” and right-sided appearance “0.” A ruler placed along the torso or spine was used to clarify laterality, in cases where the “side” of presentation was unclear. Sites of presentation were split into three groups: “Head and Neck,” “Torso,” and “Extremities.” Subjects presenting with malignancies in locations other than those listed were excluded from analysis. These definitions remained constant across analyses. Subjects presenting with multiple malignancies, discovered during initial consultation or follow-up, were recorded and closely monitored for routine inspection. Subject ethnicity was elided from this analysis.
Ordinary least squares (OLS) regression with random effects was employed to isolate the contribution of malignancy type to age at presentation, controlling for subject sex. To test associations between malignancy type and laterality, logistic regression with random effects was implemented controlling for subject sex and age. Logistic regression with random effects was performed to examine the relationship between malignancy type and site of presentation, controlling for the subject sex and age upon presentation. Finally, differences in incidence by sex were analyzed using logistic regression with random effects, controlling for subject age. Subsequent chi-squared analysis and Fisher’s Exact Test (F-Test) were undertaken to assess variable relevance when appropriate. For all analyses, Hausman testing was employed to rule-out assumption of significant endogeneity. Post-estimation Hausman testing revealed no systematic bias, indicating random effects testing was methodologically appropriate for this analysis. Seasonality of incidence was reported for reference. Statistical significance was defined as a two-sided p-value of alpha <0.05. All analyses were computed using STATA IC software.16
Results
Among those included in the cohort, BCC (54.2%) was most abundant, followed by cSCC (36.6%) and CM (9.2%). Mean age at presentation differed significantly among the trio (67.5 years + 11.7, 70.2 years + 11.1, and 60.1 years + 14.5) for BCC, cSCC, and CM respectively. Mean age at presentation for all those within the cohort was 69. Controlling for subject sex and incorporating fixed effects into OLS regression, it was determined that the diagnosis of “Melanoma” was highly significant and associated with a “younger” age at diagnosis (-8.0 years, 95% CI [-10.99 to -4.96]; p<0.0001, Table 1). cSCC, however, was associated with a significant, increased age at diagnosis (3.7 years, 95% CI [1.87 to 5.52]; p<0.0001, Table 1). No such associations were observed between BCC and age (-0.80 years, 95% CI [-2.6 to1.0]; p=0.40, Table 1).

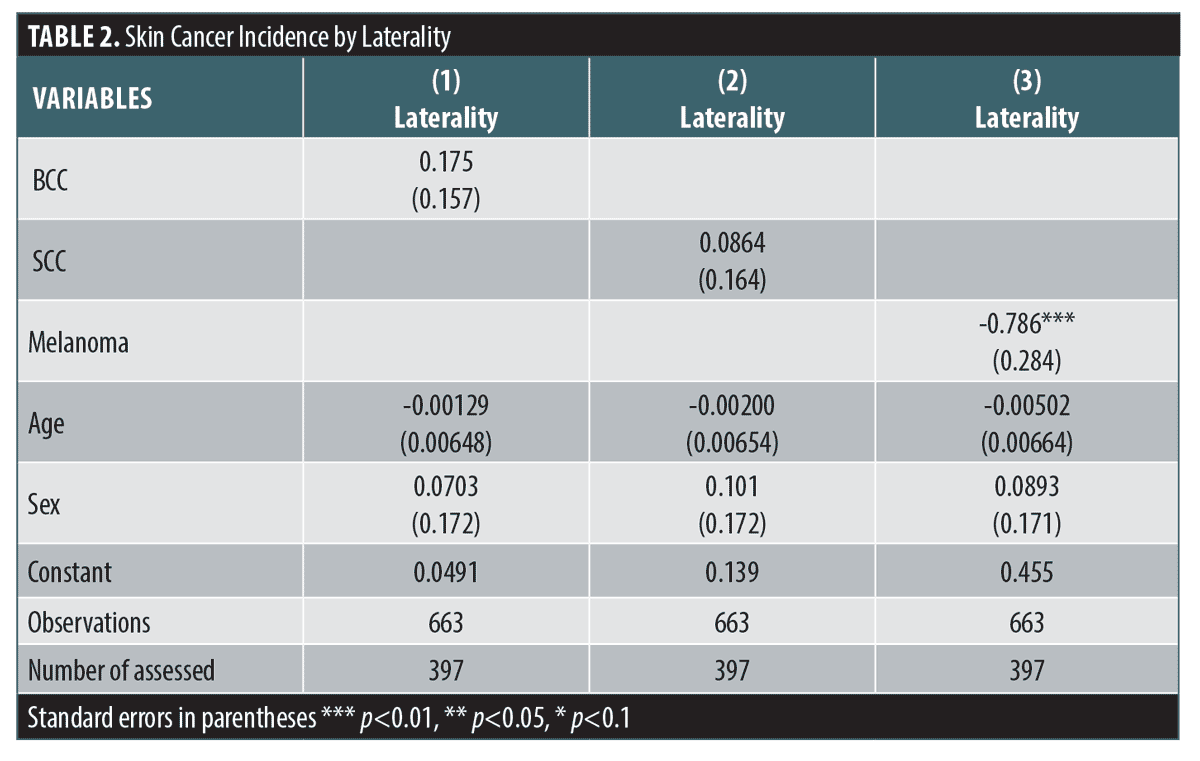
Laterality of incidence varied significantly by malignancy type. BCC (-0.18, 95% CI [-0.13 to 0.48]; p=0.27, Table 2), nor cSCC (0.09, 95% CI [-0.24-0.41]; p=0.60, Figure 2), exhibited a lateral preference for either the subjects’ left or right side. CM, however, was right-side preferring; that result was highly significant (-0.8, 95% CI [-1.3 to -0.2]; p<0.0001, Figure 2). It should be noted that post-estimation chi-squared analysis reaffirmed significance (p>chi2=0.047, Figure 2).
Malignancy type was significantly associated with the site of presentation across a variety of conditions. With respect to presentation of the “Head and Neck,” BCC exhibited a site preference (1.2, 95% CI [0.76-1.62]; p<0.0001, Table 3), whereas cSCC (-0.50, 95% CI [-0.86 to -0.05]; p=0.03, Table 3) and Melanoma (-3.5, 95% CI [-4.81 to -2.21]; p<0.0001, Table 3) exhibited significant but inverse associations with the site of head and neck. An opposite result was observed for extremities. cSCC (1.3, 95% CI [0.90 to 1.78]; p<0.0001, Table 3) and Melanoma (0.61, 95% CI [0.02 to 1.2]; p=0.05, Table 3) were site preferring in the with respect to extremities. An inverse association between the site “extremities” and BCC was observed (-1.5, 95% CI [-2.0 to -1.1]; p<0.0001, Table 3). cSCC and Melanoma exerted competing effects in the site “Torso.” Melanoma was strongly torso-site-preferring, and the relationship was highly significant (1.6, 95% CI [0.89 to 2.3]; p<0.0001, Table 3). cSCC incidence was inversely correlated with Torso (-1.0, 95% CI [-1.5 to -0.55]; p<0.0001, Table 3). No relationship was observed between BCC and Torso (0.25, 95% CI [-0.17 to 0.66]; p=0.24, Table 3).

Significant sex-specific differences were observed among the trio. Male subjects were significantly more likely to present with BCC (0.53, 95% CI [0.16 to 0.90]; p=0.006, Table 4). Female subjects, in contrast, were more likely to present with cSCC (-0.54, 95% CI [-0.92 to -0.16]; p=0.005, Table 4). No statistically significant association was observed with respect to sex and Melanoma presentation (-0.07, 95% CI [-0.70 to 0.57]; p=0.84, Table 4).

Seasonality of presentation did not differ markedly from 2017-2019 with ~10.7% of cases presenting in the summer months (May-August) and ~12.9% of cases presenting in the winter months (November-February).
Discussion
Our results relay findings from a 397 subject cohort across a range of indicators including age, laterality of incidence, site of presentation, and sex differences by malignancy type. The preponderance of our results largely accord with accepted findings. With regard to age, younger age at diagnosis for CM and advanced age at diagnosis for cSCC are in keeping with previous analyses of age-related incidence.3,4 In terms of body-site, other reviews have reported a head and neck preference for BCC and cSCC jointly,9,10 but not for melanoma.9 While cSCC did not exhibit a site preference for the head and neck, our results align with generally accepted findings with respect to BCC incidence and its preference for the head and neck—a finding thought secondary to lifetime UV burden. CM and cSCC were site preferring with respect to the extremities and torso; likewise, our findings are largely mirrored across modern cohort studies.10,11 Melanoma’s preference for the torso—among other intermittently sun-exposed areas—is well-documented and replicated across studies.12-14 Sex differences segmented by malignancy were mostly unremarkable; BCC’s male bias is well-documented in the literature.15,16
In spite of general agreement, several novel findings were observed: (1) the percentage of subjects presenting with CM was demonstrably higher than population averages would estimate;2 (2) melanoma exhibited a pronounced right-side bias; (3) cSCC was not head and neck preferring as other reviews have documented;11,20 (4) cSCC exhibited greater female bias.
Our analysis demonstrated a disproportionate incidence of CM. Estimates of non-melanoma skin cancers (NMSCs) were concomitant with population derived estimates of NMSC frequency;1 cutaneous melanoma incidence was nearly eight- to nine-fold higher than expected, according to the 2020 American Cancer Society’s annual report on cancer incidence domestically.2 It is plausible that location-dependent effects may explain this finding. Though all subjects were residents of Los Angeles at the time of presentation, ~94% were living in and around Manhattan Beach, Redondo Beach, Hermosa Beach, and Torrance, California; beach suburbs such as those listed above feature greater numbers of sun-centric outdoor activities and are “recognized” vacation destinations. Though limited analysis has been conducted on the matter, preliminary reports have suggested that sun-protective behaviors are markedly lower among residents of southern California and total UV burden is significantly higher, especially among athletes.35 We cautiously note that residents are likely to accumulate, on average, greater total solar exposure than other aged-matched controls.
Accordingly, in such regions, we can expect an outsized incidence of skin cancer, adjusted for population size, in tandem with an increase in specific skin cancers that may arise from routine, intermittent sun-exposure, such as CM.7,12 Expectedly, regions that boast pronounced solar irradiation, such as Australia and New Zealand, feature the highest rates of skin cancer globally.22 Furthermore, analysis of the area’s UV burden supports the idea that enhanced UV exposure is a plausible explanation for disproportion incidence of CM. The UV Index for the beach region’s collectively averages 10 (10/11) or “very high sun exposure risk” during the summer months (May-August) and 5.3 (5.3/11) or “moderate sun exposure risk” the remainder of the year (September-April).19 At a UV Index of 10, acute sun burn would be expected to develop in 54 percent of Fitzpatrick Phototype I subjects, and 23 percent of photo-type II subjects after ~30 minutes of direct exposure.33
Of note, the relationship between UV exposure and CM incidence is complex and subject to nuance.36, 37 While solar irradiation has been causally linked to CM incidence,25 the precise mechanisms driving melanogenesis are unresolved. We remark that the disproportionate incidence of CM, especially among non sun-exposed and intermittently sun-exposed sites, adds credence to theories which promote systemic effects associated with solar irradiation such as immunosuppression and immune-modulatory mechanisms. In particular, it has been observed that UV irradiation alters immune-surveillance, depressing prevalence and functionality of dendritic and Langerhans presenting cells.36-38 Such an outcome may impair innate anti-tumor responses mediated by pro-inflammatory cytokines (e.g. INF-gamma). Moreover, there is evidence to suggest that local irradiation may enhance the Warburg effect and promote invasion via matrix metalloproteinase (MMP) activity. In short, the contribution of sunlight to CM incidence may be mediated, in part, by the systemic modulation of immune function.
The significance of left-sided excess in the presentation of skin cancer is a contested finding with several analyses reporting contradictory results.6,8,21,25 These results compare and contrast previous findings which document marked left-sided incidence or report side-agnostic findings.5-8 In this study, we observed right-sided excess. This finding appears to be novel. It is unlikely that this result is due to chance; standard errors were robust, random effects employed, and post-estimation testing affirmed our approach. Explanations for left-sided excess tend to invoke automotive driving behaviors21,26 and constitutional factors such as sex-specific differences in nevus colonization.27,28 Importantly, we remark that while an overall finding of right side excess is novel, others have specifically demonstrated that invasive cases of CM exhibit right-side preference as well as CM among certain sub-groups, such as women over 50.21 In fact, this cohort featured a substantial share of invasive to in-situ cases (~41% invasive cases of CM). Similarly, the average age (67.9) at diagnosis for a female subject with right-sided CM was significantly greater than the cohort average for CM (60.1), though the subject “n” was underpowered to detect significant differences in age between left versus right CM presenting females. Thus, we speculate that this finding may be explained by the composition of our cohort and the specific severity of cases upon presentation.
Our analysis noted that cSCC was not explicitly head and neck preferring, a finding that opposes currently accepted site-specific patterns of cSCC incidence.11,18,29,30 While the finding is significant, we note that it is plausibly explained by developing trends in the development of NMSC by body-site, reporting a shift away from the head and neck towards other sites such as the torso and extremities, especially in female subjects.4,17,31 It appears prudent for future studies to explore the causes of this shift, but contributing factors may include differences in sun-exposure patterns, the development and use of broad-spectrum formulations of sunscreen,25 and the reduced incidence of tanning-bad consumption among high risk-groups.32 Concerning the cSCC predominance among females, rates of cSCC may be increasing in this group and thus our findings may reflect idiosyncratic cohort composition. Ultraviolet radiation is still considered chief among inciting factors in the induction of cSCC, independent of genetic variability such as TP53 mutation.30 Our subjects were likely to have high UV burden, thus providing a plausible explanatory factor for significantly increased incidence in sun-exposed sites.
With respect to sex-incidence, cancers of the skin are typically associated with males. Thus, any finding relaying female-bias is at odds with present consensus.17 We remark that while male-bias is accepted in the literature, marginal rates of increase with respect to non-melanoma skin cancers (NMSCs) is greatest for women across several age groups.17 Moreover, it has been demonstrated that while males bear outsized skin cancer burden overall, there is a significant bias with respect to female presentation among younger age-groups;18,40 other cohort studies, such as a recent exploration of cSCC and BCC incidence, have documented significant bias with respect to BCC in female subjects x > 65 years of age.40 Explanatory factors for this phenomenon have included differences in “tanning behavior,” history of oral contraceptive use, perhaps in dose-dependent fashion (though OC use and duration as a risk factor for cancers of the skin is an unresolved question) and estrogen status.25 It may be of note that disproportionate incidence of BCC:cSCC takes affect several years after menarche and then wanes a decade after menopause, reaching nearer-to-parity with male subjects.41
Conclusion
In this study, we documented insights from 663 cases (397 unique subjects) across a range of factors including age, laterality, site of presentation, and sex specific differences in incidence. The results of our analysis generally accord well with previous findings, replicating several of the most prominent results. Instances of disagreement were elucidated and theoretical explanations for our findings were discussed.
Acknowledgments
The authors thank Kristie Murias, Kellie Buglione, Jennifer Colon, Rahmi Çemen, PhD, and Summil Valdes for their technical assistance.
References
- Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the US Population, 2012. JAMA Dermatol. 2015;151(10):1081–1086.
- Cancer Facts & Figures 2020. (n.d.). Retrieved from https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html
- Lideikait? A, Moz?raitien? J, Letautien? S. Analysis of prognostic factors for melanoma patients. Acta Med Litu. 2017;24(1):25-34.
- Incidence and trends of basal cell carcinoma and cutaneous cutaneous Squamous cell carcinoma: A population-based study in Olmsted County, Minnesota, 2000-2010. (2017). Journal of the American Academy of Dermatology, 76(6).
- Bulliard J, Ess S, Bordoni A, Konzelmann I, Levi F. Left-Sided Excess in the Laterality of Cutaneous Melanoma. Arch Dermatol. 2008;144(4):556–558. doi:10.1001/archderm.144.4.556
- Brewster DH, de Vries E. Left-Sided Excess in the Laterality of Cutaneous Melanoma. Arch Dermatol. 2008;144(9):1235.
- Elder, D.E. (1995), Skin cancer. Melanoma and other specific nonmelanoma skin cancers. Cancer, 75: 245-256.
- Brewster, D. H., Horner, M. D., Rowan, S., Jelfs, P., Vries, E. D., & Pukkala, E. (2007). Left-sided excess of invasive cutaneous melanoma in six countries. European Journal of Cancer, 43(18), 2634-2637. doi:10.1016/j.ejca.2007.09.021
- Norval M, Kellett P, Wright CY. The incidence and body site of skin cancers in the population groups of South Africa. Photodermatol Photoimmunol Photomed. 2014;30(5):262-265.
- Subramaniam P, Olsen CM, Thompson BS, Whiteman DC, Neale RE, for the QSkin Sun and Health Study Investigators. Anatomical Distributions of Basal Cell Carcinoma and cutaneous Squamous Cell Carcinoma in a Population-Based Study in Queensland, Australia. JAMA Dermatol. 2017;153(2):175–182.
- McLoone, P, McLoone, P, Imanbayev, K, Norval, M. The incidence and body site of skin cancers in the population groups of Astana, Kazakhstan. Health Sci Rep. 2018; 1:e51. https://doi.org/10.1002/hsr2.51
- Ward, W. H., & Farma, J. M. (n.d.). Cutaneous melanoma: Etiology and therapy.
- Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Expert Rev Anticancer Ther. 2010;10(11):1811-1823.
- Dennis LK. Melanoma Incidence by Body Site: Effects of Birth-Cohort Adjustment. Arch Dermatol. 1999;135(12):1553–1554. Arch Dermatol.-ISSN-0003-987x-135-12-dlt1299
- Bassukas ID, Tatsioni A. Male Sex is an Inherent Risk Factor for Basal Cell Carcinoma. J Skin Cancer. 2019;2019:8304271. Published 2019 Oct 20.
- Wu S, Han J, Li WQ, Li T, Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178(6):890-897.
- Heaton H, Lawrence N. Nonmelanoma skin cancer in women. Int J Womens Dermatol. 2018;5(1):2-7. Published 2018 Nov 27.
- Al-Dujaili Z, Henry M, Dorizas AS, Sadick NS. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3(1 Suppl):S49-S51. Published 2017 Feb 16.
- Young, A. H., Knapp, K. R., Inamdar, A., Hankins, W., and Rossow, W. B.: The International Satellite Cloud Climatology Project H-Series climate data record product, Earth Syst. Sci. Data, 10, 583–593, doi.org/10.5194/essd-10-583-2018.
- McGuire JF, Ge NN, Dyson S. Nonmelanoma skin cancer of the head and neck I: histopathology and clinical behavior. Am J Otolaryngol. 2009;30(2):121-133.
- Butler, Susan T. et al, Journal of the American Academy of Dermatology, Volume 63, Issue 6, 1006 – 1010
- Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7(2):1-6. Published 2017 Apr 30.
- Gilmore S. Melanoma screening: Informing public health policy with quantitative modelling. PLoS One. 2017;12(9):e0182349. Published 2017 Sep 25. doi:10.1371/journal.pone.0182349
- Karimkhani C, Green AC, Nijsten T, et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177(1):134-140.
- Hands, J., & Moy, L. (2020). A Review of Exogenous Factors Implicated in the Induction of Cutaneous Melanoma. SKIN The Journal of Cutaneous Medicine, 4(3), 200-220.
- Paulson KG, Iyer JG, Nghiem P. Asymmetric lateral distribution of melanoma and Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2011;65(1):35-39.
- Autier P, Boniol M, Severi G, Pedeux R, Grivegnée AR, Doré JF. Sex differences in numbers of nevi on body sites of young European children: implications for the etiology of cutaneous melanoma. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2003-2005.
- Visconti A, Sanna M, Bataille V, Falchi M. Genetics plays a role in nevi distribution in women. Melanoma Manag. 2020;7(1):MMT35. Published 2020 Mar 17.
- Ouyang YH. Skin cancer of the head and neck. Semin Plast Surg. 2010;24(2):117-126.
- Howell JY, Ramsey ML. Cancer, cutaneous Squamous Cell of the Skin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-.
- Heckman CJ, Darlow SD, Ritterband LM, Handorf EA, Manne SL. Efficacy of an Intervention to Alter Skin Cancer Risk Behaviors in Young Adults. Am J Prev Med. 2016;51(1):1-11.
- Diehl K, Görig T, Greinert R, Breitbart EW, Schneider S. Trends in Tanning Bed Use, Motivation, and Risk Awareness in Germany: Findings from Four Waves of the National Cancer Aid Monitoring (NCAM). Int J Environ Res Public Health. 2019;16(20):3913. Published 2019 Oct 15.
- Sánchez-Pérez JF, Vicente-Agullo D, Barberá M, Castro-Rodríguez E, Cánovas M. Relationship between ultraviolet index (UVI) and first-, second- and third-degree sunburn using the Probit methodology. Sci Rep. 2019;9(1):733. Published 2019 Jan 24.
- United States Census Bureau / American FactFinder. “P12 : Sex by Age.” 2010 Census.U.S. Census Bureau, 2010.Web. 1 January 2013 <http://factfinder2.census.gov>.
- Cohen PH, Tsai H, Puffer JC. Sun-protective behavior among high-school and collegiate athletes in Los Angeles, CA. Clin J Sport Med. 2006;16(3):253-260.
- Arisi M, Zane C, Caravello S, et al. Sun Exposure and Melanoma, Certainties and Weaknesses of the Present Knowledge. Front Med (Lausanne). 2018;5:235. Published 2018 Aug 30.
- Shuster S. Is sun exposure a major cause of melanoma? No. BMJ. 2008;337:a764. Published 2008 Jul 22.
- Arisi M, Zane C, Caravello S, et al. Sun Exposure and Melanoma, Certainties and Weaknesses of the Present Knowledge. Front Med (Lausanne). 2018;5:235. Published 2018 Aug 30.
- Rünger TM. Mechanisms of Melanoma Promotion by Ultraviolet Radiation. J Invest Dermatol. 2016;136(9):1751-1752.
- Lukowiak TM, Aizman L, Perz A, et al. Association of Age, Sex, Race, and Geographic Region With Variation of the Ratio of Basal Cell to Cutaneous Squamous Cell Carcinomas in the United States. JAMA Dermatol. 2020;156(11):1192–1198.
- Lukowiak, T. M., Aizman, L., Perz, A., Etkzorn, J., Miller, C., Shin, T., …Lee, M. P. (2020). 400 Rates of BCC relative to SCC are higher in younger patients, especially females. Journal of Investigative Dermatology, 140(7), S52.

