 J Clin Aesthet Dermatol. 2023;16(7):63–65.
J Clin Aesthet Dermatol. 2023;16(7):63–65.
by Jerome Kaikati, MD; Nizar El Bcherawi, MD; Jad Abou Khater, MD; Serena Maria Dib, MD; Elio Kechichian, MD; and Josiane Helou, MD
All authors are with the Dermatology department and Faculty of Medicine at Saint-Joseph University in Beirut, Lebanon. Additionally, Dr. Helou is a Professor in the Chief of the dermatology department and Faculty of Medicine at Saint-Joseph University in Beirut, Lebanon.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Melasma is a widespread condition that affects people of many ethnicities and is prevalent in the Middle East. To date, the therapeutic arsenal is still not effective, especially in countries with high ultraviolet light index. New treatment options are needed.
Objective. The aim of this pilot study was to assess the efficacy of topical tranexamic acid (TA) 2% combined with vitamin C 2% in the treatment of resistant melasma in the Mediterranean region.
Methods. This prospective interventional pilot study included 10 women, aged 18 to 55 years, with resistant melasma. Intervention consisted in application of a topical formulation containing 2% TA and 2% vitamin C, every night for eight weeks. The primary outcome was the Melasma Area and Severity Index (MASI) score measured at baseline and at Weeks 4 and 8. Melasma Quality of Life Scale (MelasQoL) and Physician Global Assessment (PGA) were used at baseline and at Weeks 4 and 8 of treatment, and they were set as the secondary outcomes.
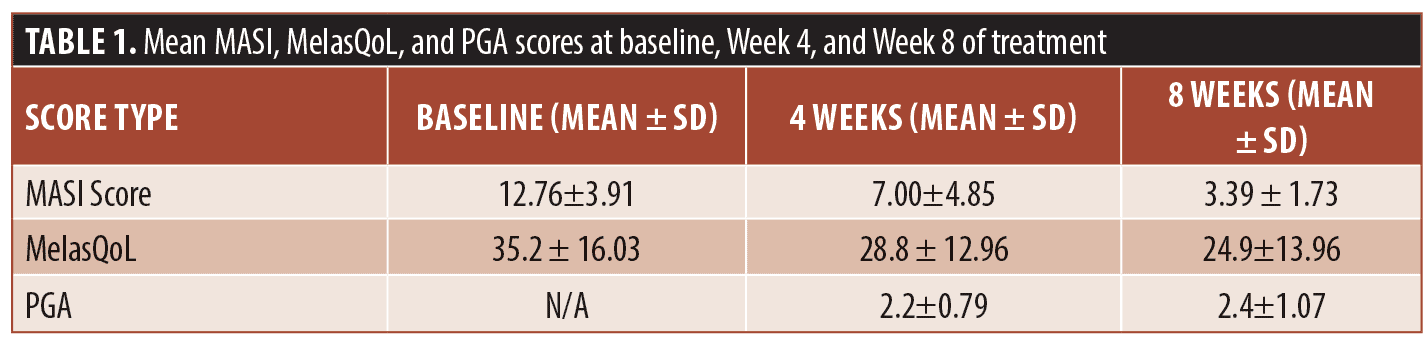
Results. The mean MASI score varied from 12.76±3.91 at baseline to 7.00±4.85 at Week 4 (p<0.01) then to 3.39 ± 1 at Week 8 (p=0.03). The mean MelasQoL decreased from 35.2 ± 16.03 at baseline to 28.8 ± 12.96 at Week 4 (p<0.01) then to 24.9±13.96 at Week 8 (p=0.14). The PGA increased between Weeks 4 and 8 passing from 2.2±0.79 to 2.4±1.07. No major side effects were reported.
Conclusion. Our pilot study demonstrated the possibility of a topical combination of TA 2% and vitamin C 2 %, which may be a useful therapeutic strategy in the treatment of resistant melasma in the Middle east, a region of the world with high UV index. This combination treatment is a safer alternative to dangerous bleaching treatments that are still being used.
Keywords. Melasma, Topical tranexamic acid, Vitamin C
Melasma is a widespread condition that affects people of many ethnicities, but it is more common in women of Hispanic, Middle Eastern, Asian, and African descent (Fitzpatrick Skin Phototypes III-V).1 To date, the therapeutic arsenal is still not entirely effective due to side effects, recurrence, and limited efficacy, especially in countries with high UV index. New and effective treatment options are needed.
Recent studies have revealed that the classic hemostatic medication tranexamic acid (TA) has a hypopigmentatory effect on melasma lesions, inhibiting UV-induced pigmentation.3 The primary mechanism of TA’s hypopigmentory action is believed to be its anti-plasmin activity.3
One of the commonly used topical treatments is vitamin C, which inhibits the tyrosinase enzyme by interacting with copper ions at the enzyme’s active site. Because vitamin C is an unstable substance, it must be paired with other depigmenting ingredients.4 The aim of our pilot study was to assess the efficacy of topical TA 2% combined with vitamin C 2% in the treatment of resistant melasma in the Mediterranean region.
Methods
A prospective interventional pilot study was conducted from February 2022 to July 2022, in Hôtel-Dieu de France University Hospital in Beirut, Lebanon. All patients provided informed consent for participating in this study, and the research project was approved by Research and Ethical committee of the hospital.
Inclusion and exclusion criteria. We included women aged 18 to 55 years with resistant melasma, living in the Middle East region. Resistance was outlined as either a recurrence of melasma following the completion of treatment or the ineffectiveness of one well-executed treatment regimen that included the use of hydroquinone or other tyrosinase inhibitors, topical tretinoin, chemical peels, laser and light therapies, or dermabrasion.
We excluded patients with history of any other depigmenting treatment in the past six weeks, pregnant or lactating females, patients on hormone replacement therapy, oral contraceptives, or photosensitizing drugs, patients with any known allergy to vitamin C, and patients with cancer.
Study design and outcome measures. Diagnosis of melasma was based on clinical evaluation by a board-certified dermatologist. Participants applied a topical formulation containing 2% TA and 2% vitamin C every night for eight weeks. The primary outcome was the Melasma Area and Severity Index (MASI) score measured by two blinded independent board-certified dermatologists at baseline and at Weeks 4 and 8. Melasma Quality of Life Scale (MelasQoL) was completed by patients to assess the impact of melasma on the quality of life, at baseline and at Weeks 4 and 8 of treatment. Physician Global Assessment PGA was also used at Weeks 4 and 8 of treatment. MelasQoL and PGA constituted our secondary outcomes. Adverse events, if present, were recorded at each visit (Week 4 and Week 8).
Data was analyzed using Statistical Package for the Social Sciences (SPSS version 18 for windows). A repeated measurement t-test was used to assess the changes in MASI and MelasQoL. Statistical significance was set at p<0.05.
Results
Through the duration of the trial, 10 women with resistant melasma were included. No patient dropped out during the duration of the study. The age of participants ranged from 28 to 65 years averaging 45.9 ± 11.3 years.
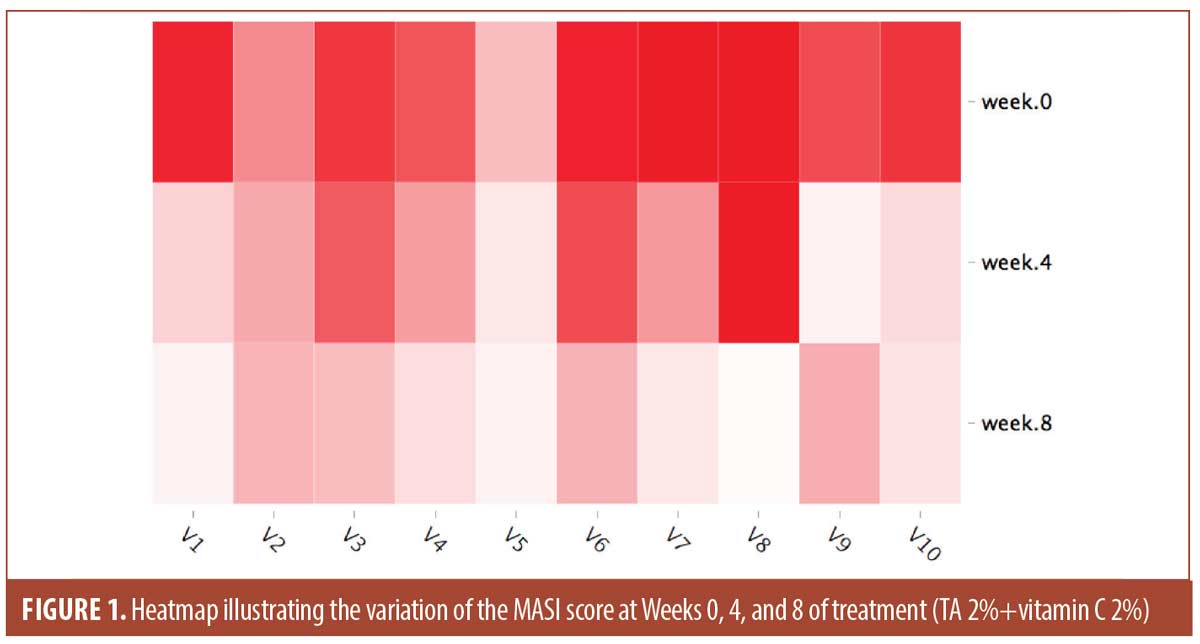
The mean values for the scores used at baseline, Weeks 4 and 8 are shown in Table 1. The mean MASI score was 12.76 ±3.91 at baseline and 3.39 ± 1 at Week 8. The evolution of the MASI Score during the trial is illustrated in Figure 1. The mean MelasQoL decreased from 35.2 ± 16.03 at baseline to 24.9±13.96 at Week 8. The PGA increased between Weeks 4 and 8 passing from 2.2±0.79 to 2.4±1.07.
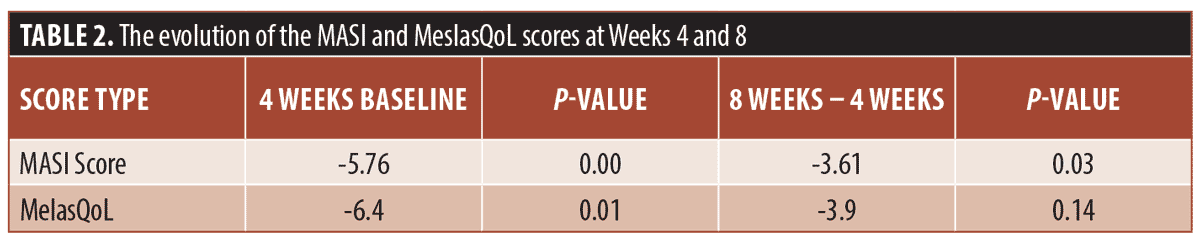
A repeated-measure t-test was performed. The results showed a significant change in MASI score when comparing baseline to Week 4 and Week 4 to Week 8. However, for the MelasQoL a significant reduction only occurred between baseline and Week 4 (Table 2). Of our 10 patients, only one reported dry skin as a side effect of the treatment, which did not lead to discontinuation of treatment.
Discussion
Despite the multiple treatment modalities of hyperpigmentation (e.g., chemical peels, laser and light therapies, dermabrasion), medical treatments continue to be a cornerstone of melasma management. Some over-the-counter cosmetic products include a topical mixture of TA and vitamin C. A topical preparation containing synthetic recombinant human growth factors, TA, vitamin C, arbutin 3%, and niacinamide 5%, has shown to be safe and effective in the treatment of hyperpigmentation.5
Based on our results, a simpler combination of TA 2% and topical vitamin C 2% offers an effective tool in treating resistant melasma, especially in the Mediterranean region, where Fitzpatrick Skin phototypes are commonly III to V, and where the UV index varies between 9 and 10. Indeed, our results showed an impressive decrease in MASI score, the most appropriate objective assessment tool. Compared to other trials, the efficacy of topical TA treatment paired with vitamin C in our cohort is greater than in others using topical TA alone. In fact, the recorded quick improvement in MASI score of 5.76 points at Week 4 is better than what was noted in other trials without vitamin C, as described in the literature (MASI 1.36 at 4 weeks).6 The combination of these two agents with low concentration did not show any alarming side effects, while achieving satisfactory results. In fact, in our study we only reported dry skin as a side effect. Trials using higher concentration of TA and vitamin C showed erythema, scaling, and mild to moderate burning.7
The use of hydroquinone (HQ) is still debated, despite its notable skin lightening effects, due of potentially carcinogenic and toxic effects.7 Topical TA formulations have shown promise as alternatives to HQ, including a 2% emulsion, a 3% cream, a 5% solution, and a 5% liposomal cream, which have all been clinically demonstrated to be beneficial in the treatment of melasma.5 Intradermal TA has also been used and has demonstrated a significant decrease in MASI.8
Furthermore, vitamin C (i.e., ascorbic acid) a well-known antioxidant, binds to copper to block tyrosinase enzyme melanogenesis pathway. When comparing its efficacy for the treatment of melasma to HQ 4%, substantial improvement was observed during the first month with HQ 4%, while results with topical ascorbic acid were not apparent until the third month of treatment.7 The intradermal injection of a combination of TA and ascorbic acid has also been shown to significantly reduce patients’ MASI scores, and this improvement was sustained for three months.8 With topical application or intradermal injection, the combination of TA with vitamin C represents a breakthrough in the management of melasma, especially where other conventional therapies have failed.
In our study, not only did the objective scores improve after the combination of TA and Vitamin C, but also subjective scales, as the MelasQoL improved significantly from 35.2 to 28.2. Improvement for the MelasQoL was not significant when comparing Weeks 4 to 8 while it was significant for the MASI Score. Patients might have perceived improvement as more drastic at the beginning of treatment with stabilization towards the second month of treatment. Additional demographic profile of patients that takes into account their marital status, socioeconomic status, and history of psychiatric illness may help to establish the variables that affect MelasQoL in Middle Eastern women. This has been discussed in other parts of the world, such as in Singapore.9 Populational prevalence of melasma varies according to ethnic composition, skin phototype, and intensity of sun exposure.1 The impact of melasma on quality of life is certainly related to cultural and societal factors and is not necessarily related to the melasma severity (MASI).2 Compared to European countries like France, the middle east population seems to be more affected by the disease as shown in our study where the MelasQoL mean at baseline was 35.2 compared to 20.0 in Misery et al study.10
A study conducted in an Arab population outside of the Middle East investigated how the Arab American population feels about skin perception.11 While more recent immigrants to the United States tended to prefer very fair or fair skin, those who had been in the country for a longer period of time were more inclined to find olive or dark skin to be more attractive. This can be explained by the fact that having light skin is seen as a sign of beauty and prestige in the Middle East. Alrayyes et al12 showed that Saudi women frequently use skin-lightening creams because of societal ideals of beauty and social advantage. To the best of our knowledge, in this region of the world where physical appearance is highly valued, this is the first study that evaluates the impact of resistant melasma on quality of life and its evolution following the combination treatment with topical TA and vitamin C.
Conclusion
Despite the serious problems that hyperpigmentation can create in the Middle East, skin lightening treatments nevertheless can contain harmful ingredients, and are still accessible over the counter due to consumer demand and practical considerations. In reality, hydroquinone-based products, topical application of mercury or a heavy element like arsenic, are unfortunately still used in Arab countries.12 The cosmetic use of hydroquinone is prohibited in regions like Europe, where it can only be obtained with a prescription. However, it is still found in cosmetic products in countries like Saudi Arabia and Lebanon, even at concentrations above 2%.12 Finding an effective and safe option for the treatment of melasma is crucial, particularly in this area, where UV index is high, and melasma is resistant/recurrent. Our pilot study demonstrated the efficacy of a topical combination of TA 2% and vitamin C 2 %, which may be a useful therapeutic strategy. Many side effects of conventional melasma therapies, especially photosensitivity in this bright region of the world, are avoided by this combination. Our investigation is certainly limited by the sample size. Larger studies should be conducted in the region to better define alternative to dangerous bleaching treatments that are still being used recklessly.
References
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89(5):771–782.
- Zhu Y, Zeng X, Ying J, et al. Evaluating the quality of life among melasma patients using the MELASQoL scale: A systematic review and meta-analysis. PLoS One. 2022;17(1):e0262833.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014 Aug;19(8):753–757.
- Ismail ESA, Patsatsi A, Abd El-Maged WM, et al. Efficacy of microneedling with topical vitamin C in the treatment of melasma. J Cosmet Dermatol. 2019 Feb 15;
- Kalasho BD, Minokadeh A, Zhang-Nunes S, et al. Evaluating the Safety and Efficacy of a Topical Formulation Containing Epidermal Growth Factor, Tranexamic Acid, Vitamin C, Arbutin, Niacinamide and Other Ingredients as Hydroquinone 4% Alternatives to Improve Hyperpigmentation: A Prospective, Randomized, Controlled Split Face Study. J Cosmet Sci. 2020 Oct;71(5):263–290.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and Safety of Tranexamic Acid in Melasma: A Meta-analysis and Systematic Review. Acta Derm Venereol. 2017 Jul 6;97(7):776–781.
- González-Molina V, Martí-Pineda A, González N. Topical Treatments for Melasma and Their Mechanism of Action. J Clin Aesthet Dermatol. 2022 May;15(5):19–28.
- Pazyar N, Molavi SN, Hosseinpour P, et al. Efficacy of intradermal injection of tranexamic acid and ascorbic acid versus tranexamic acid and placebo in the treatment of melasma: A split-face comparative trial. Health Sci Rep. 2022 Mar;5(2):e537.
- Harumi O, Leok Goh C. The Effect of Melasma on the Quality of Life in a Sample of Women Living in Singapore. J Clin Aesthet Dermatol. 2016 Jan;9(1):21–24.
- Misery L, Schmitt AM, Boussetta S, et al. Melasma: measure of the impact on quality of life using the French version of MELASQOL after cross-cultural adaptation. Acta Derm Venereol. 2010 May;90(3):331–332.
- El-Essawi D, Musial JL, Hammad A, et al. A survey of skin disease and skin-related issues in Arab Americans. J Am Acad Dermatol. 2007 Jun;56(6):933–938.
- Alrayyes SF, Alrayyes SF, Farooq Dar U. Skin-lightening practices behind the veil: An epidemiological study among Saudi women. J Cosmet Dermatol. 2020 Jan;19(1):147–153.

