 J Clin Aesthet Dermatol. 2021;14(11):18–25.
J Clin Aesthet Dermatol. 2021;14(11):18–25.
by Antoine Salloum, MD; Jenna Koblinski, MD; Nagham Bazzi, MD; and Nathalie C. Zeitouni, MDCM, FRCPC
Dr. Salloum is with the Department of Dermatology at Saint George Hospital University Medical Center in Beirut, Lebanon, and Dermatologic Surgicenter in Philadelphia, Pennsylvania. Drs. Koblinski and Zeitouni are with the University of Arizona College of Medicine in Phoenix, Arizona. Dr. Bazzi is with the Faculty of Medicine at Lebanese University in Beirut, Lebanon.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Talimogene laherparepvec (T-VEC) is the first oncolytic virus therapy approved by the United States Food and Drug Administration (in 2015) for the treatment of advanced-stage melanoma. Despite a paucity of Phase III trials for T-VEC as a therapy for non-melanoma cancers, successful off-label use of T-VEC for this purpose has been reported in the literature.
Objective. We sought to review the literature describing T-VEC as a treatment for non-melanoma cancer.
Methods. Systematic searches of the PubMed literature database and ClinicalTrials.gov website were performed in July 2020, focusing on T-VEC in combination with non-melanoma cancer, including squamous cell carcinoma, Merkel cell carcinoma, sarcoma, cutaneous B-cell lymphoma, and cutaneous T-cell lymphoma. Articles were screened based on their title and abstract.
Results. Nine articles with 87 patients were included. Relevant articles included case reports, case series, and Phase I and Phase II trials. The majority of patients in the studies had refractory cancers or had been heavily pretreated. Overall, T-VEC demonstrated efficacy for non-melanoma cancer, both independently and in combination with biologics.
Conclusion. T-VEC has demonstrated efficacy for non-melanoma cancers. Phase III trials of T-VEC for this indication are warranted to expand its clinical utility.
Key words: Talimogene laherparepvec, non-melanoma cancer, squamous cell carcinoma, Merkel cell carcinoma, sarcoma, cutaneous B-cell lymphoma, cutaneous T-cell lymphoma
______________________________________________________________________
Talimogene laherparepvec (T-VEC) is the first oncolytic virus therapy that was approved by the United States Food and Drug Administration (FDA) (in 2015) for the treatment of advanced-stage melanoma. T-VEC is administered through intratumoral injection and has been demonstrated to be effective and well-tolerated in patients with melanoma.1–4 The Phase III randomized, controlled study, OncovexGM-CSF Pivotal Trial in Melanoma (OPTiM) compared intralesional T-VEC to granulocyte-macrophage colony-stimulating factor (GM-CSF). This trial found that the T-VEC–treated group had a significantly higher durable response rate (continuous response lasting at least 6 months) in comparison to the GM-CSF-treated group.5 Based on the OPTiM findings, T-VEC received FDA approval for melanoma and has since been used in clinical practice as both a monotherapy as well as off-label in combination with other immunotherapies, radiation therapy, and chemotherapy.1–5 Since the FDA approved T-VEC as a melanoma treatment, its efficacy has continued to be explored. One study of T-VEC use in 80 patients with melanoma by Louie et al6 found complete local response in 39 percent of patients and partial response in 18 percent; in addition, at the last follow-up, 37 percent of the complete responders had no evidence of disease. This study also further demonstrated the safety and tolerability of T-VEC while also confirming its utility across multiple anatomic locations.6
Oncolytic viruses selectively target cancer cells, and their replication within these cells induces tumor cell lysis. This function can be attributed to either intrinsic viral properties or engineered alterations to the virus. T-VEC is a herpes simplex virus type 1 (HSV-1) that has been modified through the deletion of infected cell protein (ICP) 34.5 and ICP47 genes as well as through insertion of human GM-CSF. Each of these changes help lead to the therapeutic effects of T-VEC. The selective targeting of cancer cells is accomplished through the deletion of ICP34.5, whereas the deletion of ICP47 yields improved antigen presentation of HSV-infected cells. Finally, the incorporation of GM-CSF to the virus induces a systemic immune response. In addition to contributing to tumor cell lysis of injected cells, this systemic effect is also thought to affect distant uninjected tumor sites.1,2,4,7
Currently, T-VEC is only FDA-approved for Stage IIIB through IV melanoma.4 This is due to a lack of Phase III clinical trials evaluating T-VEC efficacy for non-melanoma cancers. However, despite this paucity of Phase III trials, successful off-label use of T-VEC for non-melanoma cancers has been reported in the literature. This includes Merkel cell carcinoma (MCC), sarcoma, squamous cell carcinoma of the head and neck (SCCHN), and lymphoma.8–16 These reports provide insight into the potential benefits of T-VEC as a more widely used therapy. We performed a systematic review to synthesize the findings of these studies and to hopefully serve as a stepping stone towards Phase III clinical trials evaluating efficacy of T-VEC for non-melanoma cancers.
Methods
Eligibility criteria. Original English case reports, case series, and clinical trials were eligible if they were published in a peer-reviewed journal and reported on human patients. Non-case reports, such as reviews, basic research, or commentaries were excluded. Articles assessing T-VEC as a treatment for melanoma cancer were excluded.
Search methods. A priori protocol was approved by all authors. On July 18, 2020, a literature search was conducted and focused on T-VEC drug use in non-melanoma cancer. The PubMed database and ClinicalTrials.gov website were searched using various combination of the following free text: “T-VEC in squamous cell carcinoma,” “T-VEC in Merkel cell carcinoma,” “T-VEC in sarcomas,” T-VEC in cutaneous B cell lymphoma,” and “T-VEC in cutaneous T-cell lymphoma.” Articles and trials were evaluated by the authors (A. S. and N. B.), and the most relevant articles were selected according to their titles and abstracts. All articles that pertained to the subject and were published before the search date were included in the systematic review.
Reference lists of identified original articles or reviews were searched manually. The stepwise approach is shown in Figure 1.
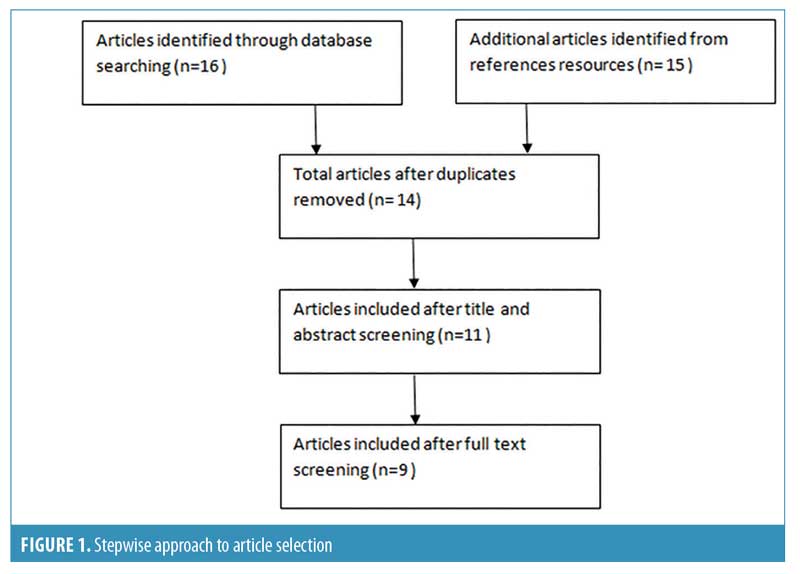
Results
Nine articles with 87 patients were included in the study. Three articles were case reports,8,9,14 two were case series,10,11 two were Phase I studies,13,16 one was a Phase I/II study,15 and one was a Phase II study.12 The mean age of the included patients was 58.3 years old. Eight patients had MCC,8–11 eight patients had locally advanced sarcoma,12 12 patients had metastatic sarcoma,12 58 patients had SCCHN,13,15,16 and one patient had concomitant primary cutaneous anaplastic large cell lymphoma (ALCL) and metastatic melanoma.14
Fourteen clinical trials were currently underway studying the effects of T-VEC on non-melanoma cancer, and the majority are Phase II trials. Four clinical trials are combining T-VEC with radiation, two are combining it with nivolumab, and two are combining it with pembrolizumab.
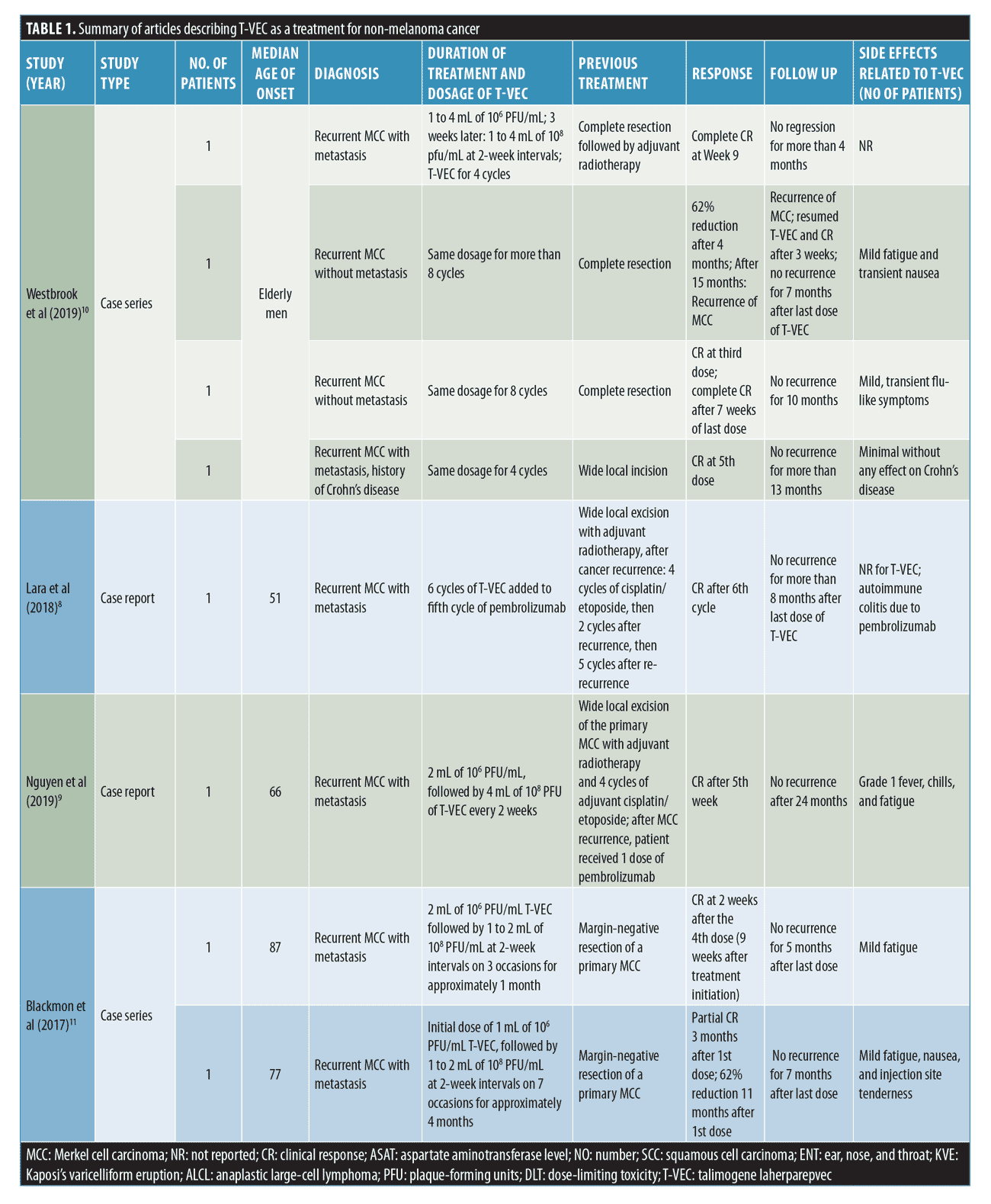
MCC. Eight patients had recurrent MCC, including six with metastasis.8–11 The mean age of these patients was 70.2 years old.8–11
Almost all of the patients (n=7, 87.5%) underwent complete resection or wide local excision of the primary MCC, which recurred in all patients. Among them, 25% (n=2) of patients received 4 to 11 cycles of cisplatin/etoposide, and 37.5% (n=3) received adjuvant radiotherapy. After recurrence of the disease, T-VEC was administered.8–11
The first dose of T-VEC ranged between 1 and 4 mL of 106 PFU/mL. Further dosages ranged between 1 and 4 mL of 108 plaque-forming unit (PFU)/mL.8–11 In the case report by Lara et al,8 the patient was started on salvage programmed cell death protein 1 therapy with nivolumab for his MCC. The nivolumab was discontinued after two cycles due to development of autoimmune diabetes. The patient subsequently began pembrolizumab, with T-VEC being added to the fifth cycle of pembrolizumab. The patient underwent six cycles of T-VEC with a clinical response (CR) resulting in no residual nodules to inject. The patient then continued solely with pembrolizumab.8
All patients had a CR,8–11 including two with a complete CR (CCR).10
The majority of patients did not have recurrent MCC for at least four months of follow-up.8–11 One patient had a recurrence of MCC and resumed T-VEC without further recurrence.10
Adverse events (AEs) reported in the course of treatment with T-VEC included mild fatigue in 50% (n=4), nausea in 25% (n=2), flu-like symptoms in 12.5% (n=1), and injection site tenderness in 12.5% (n=1). T-VEC injections did not have any effect on Crohn’s disease, and no relapse was seen during the drug-intake period.8–11
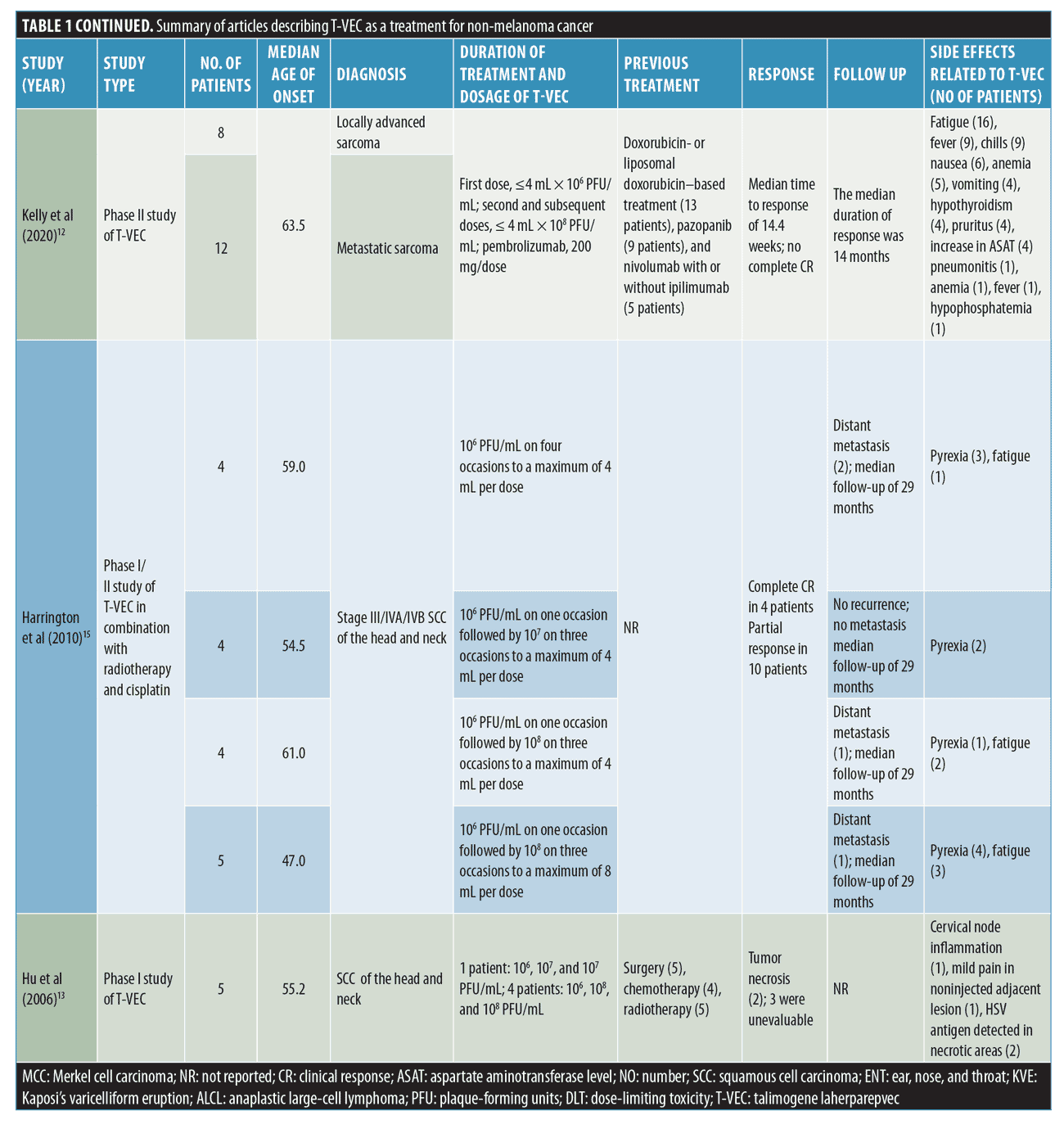
Sarcoma. Twenty patients had locally advanced sarcoma or metastatic sarcoma. The mean age of these patients was 63.5 years old.12
A total of 13 patients (65%) had previously received doxorubicin- or liposomal doxorubicin–based treatment, and nine patients (45%) had received pazopanib. Immunotherapy (nivolumab with or without ipilimumab) was prescribed in five patients (25%).12
All study participants received both T-VEC and pembrolizumab on Day 1 of each 21-day cycle. For T-VEC dosing: the first dose of T-VEC was less than 4 mL × 106 PFU/mL, and the second and subsequent doses were equal or less than 4 mL × 108 PFU/mL. The pembrolizumab dose was 200 mg/dose.12
No CCRs were noted. The median time to partial CR was 14.4 weeks. Follow-up was uneventful for 14 months.12
AEs reported included fatigue in 80 percent (n=16), fever in 45 percent (n=9), chills in 45 percent (n=9), nausea in 30 percent (n=6), anemia in 25 percent (n=5), vomiting in 20 percent (n=4), hypothyroidism in 20 percent (n=4), pruritus in 20 percent (n=4), increase in aspartate aminotransferase (AST) in 20 percent (n=4), pneumonitis in 5 percent (n=1), anemia in 5 percent (n=1), fever in 5 percent (n=1), and hypophosphatemia in 5 percent (n=1).12
SCCHN. A total of 58 patients had SCCHN.13,15,16 Of these patients only five SCCHN were from the study by Hu et al.13 Of those five patients, only two were evaluable. Among the other three patients, one was unable to have a final biopsy and the other two were non-evaluable due to disease progression.13 The mean age of these patients was 55.4 years.13,15,16
Prior treatments included chemotherapy in 64.5 percent (n=40), radiotherapy in 61.3 percent (n=38), immunotherapy in 4.8 percent (n=3), targeted biologics in 9.6 percent (n=6), and surgery in 8 percent (n=5) of patients, respectively.13,15,16
A total of 21 patients received radiotherapy followed by T-VEC injection, then cisplatin chemotherapy. Patients were admitted to the hospital for 48 hours and were screened for JS1/34.5-/47-/GM-CSF. Patients were discharged after a negative swab test.15
The first dose was 106 PFU/mL,13,15,16 and the second dose ranged between 106 and 108 PFU/mL.13,15,16
A CCR was seen in 6.4 percent of patients (n=4), a partial response was seen in 16.1 percent (n=10), and tumor necrosis was seen in 3.2 percent of patients (n=2).13,15,16
Patients were followed for 29 months. Distant metastasis of SCCHN was seen in 6.4 percent (n=4) of patients.13,15,16
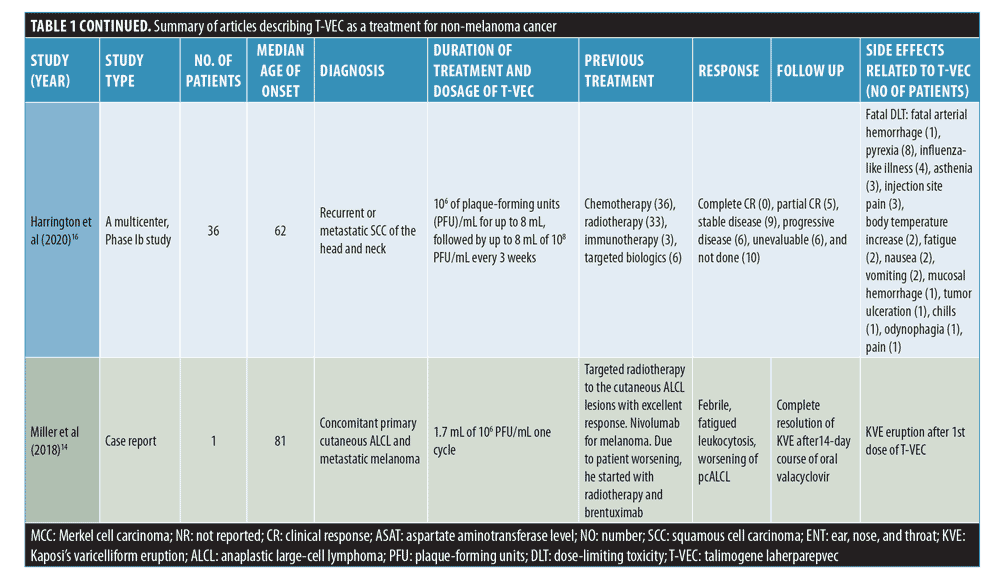
AEs included fatal arterial hemorrhage (n=1), pyrexia (n=18), HSV antigens detected in necrotic areas (n=2), fatigue (n=8), cervical node inflammation (n=1), mild pain in uninjected adjacent lesion (n=1), pain (n=1), flu-like illness (n=4), asthenia (n=3), injection site pain (n=3), body temperature increase (n=2), nausea (n=2), vomiting (n=2), mucosal hemorrhage (n=1), tumor ulceration (n=1), chills (n=1), and odynophagia (n=1).13,15,16
Primary cutaneous ALCL. One patient (81 years old) had concomitant primary cutaneous ALCL and metastatic melanoma. He first received targeted radiotherapy to the cutaneous ALCL lesions with an excellent response as well as nivolumab for his melanoma. However, his condition subsequently worsened, and he was started on radiotherapy with brentuximab. The patient had further complications and due to his severe medical conditions, treatment options were limited. On Cycle 4, Day 1 of nivolumab, he started his first dose of T-VEC (1.7 mL of 106 PFU/mL). The patient developed a fever and Kaposi’s varicelliform eruption after the first dose. T-VEC was then stopped and oral valacyclovir was prescribed. After 14 days, a complete resolution of the Kaposi’s varicelliform eruption was noted.14
Discussion
Oncolytic virus therapy is an active area of research and the advent of intralesional T-VEC has advanced the treatment of melanoma. T-VEC is currently under investigation for non-melanoma cancer as well, and this systematic review synthesizes current data on the topic. The majority of the patients in the studies had refractory cancers or had been heavily pretreated.8–15 The positive results of T-VEC in light of these previously failed therapies is encouraging.
In our review, we found that all patients with MCC had a CR to T-VEC, with a small subset of patients having a CCR. While there were only eight patients in the MCC cohort, this is promising for T-VEC’s potential as an MCC therapy.8–11 MCC shares some similarities with melanoma, as they are both aggressive cutaneous cancers correlated with ultraviolet exposure and immunosuppression,17–19 and this may be a contributing factor to the apparent success of T-VEC for MCC as T-VEC has proven benefit for melanoma.1,4,5
There were three studies involving T-VEC therapy for SCCHN.13,15,16 In the Phase I study by Hu et al,13 there were only five patients with SCCHN; the remaining 25 patients had subcutaneous metastases from the breast, gastrointestinal adenocarcinoma, or malignant melanoma. Out of the five participants with SCCHN, two were non-evaluable due to disease progression, and one was non-evaluable because a final biopsy was not obtained. This study found no CRs or partial responses in any of the evaluated patients, though stable disease and flattening/shrinkage of the injected lesions were found in some of the non-SCCHN malignancies.13 However, in the 2010 Phase I/II study by Harrington et al,15 82.3 percent of patients had a tumor response and 93 percent of patients had pathologic complete remission at neck dissection. In the 2020 Phase Ib study by Harrington et al,16 a confirmed partial response was observed in 13.9% of patients. The variability in findings between the Hu et al13 and both Harrington et al15,16 studies highlights the need for more research to evaluate T-VEC’s potential for SCCHN. Furthermore, in the study by Hu et al,13 there were only two patients with SCCHN evaluated, which is a very small sample size. In addition, participants in this study were only followed for six weeks post-final injection, which might have contributed to the lack of CR observed.13 Both of the studies by Harrington et al15,16 demonstrated the potential of T-VEC as a therapy for SCCHN, and this might suggest the prospective use of T-VEC for other squamous cell cancer types as well, such as cutaneous SCC. Currently, the University of Arizona is completing a Phase II, single-arm clinical trial evaluating T-VEC as a monotherapy for low-risk cutaneous SCC, with an estimated completion date of 2026.20 Findings from this trial will continue to move T-VEC’s function forward.
Upon review of the literature, there was one study found that looked at T-VEC’s utility for sarcoma treatment; this was a Phase II study by Kelly et al12 that used a combination of T-VEC and pembrolizumab. The study demonstrated an objective response rate of 35 percent. While no CCR was noted, their findings are promising for sarcoma treatment, as chemotherapy (the current mainstay of therapy for advanced disease) has an objective response rate of approximately 20 percent.12,21 This favorable outcome for sarcoma should be further evaluated, and Kelly et al have reported they are currently in the process of evaluating T-VEC with pembrolizumab for angiosarcoma, undifferentiated pleomorphic sarcoma, myxofibrosarcoma, and epithelioid sarcoma.12
While many of these studies demonstrate success of T-VEC in non-melanoma cancer treatment, it is important to note the AEs associated with T-VEC therapy. During our literature review, the most common adverse effects were flu-like symptoms, including grade I fever, mild fatigue, and transient nausea, which are also reported in the literature during T-VEC use in melanoma patients.8–15,22 Other AEs were observed throughout the different studies as well. In the systematic review, there was one patient being treated for concomitant primary cutaneous ALCL and metastatic melanoma who had to stop his T-VEC therapy due to developing a Kaposi’s varicelliform eruption along with worsening of his ALCL.14 This demonstrates that patients should be counseled to report any new rashes or lesions to their health care practitioner. Because this patient had two cancer types, it is unclear how T-VEC might affect lymphoma independently, and further studies without this confounder are necessary. Overall however, T-VEC is well-tolerated by patients.4 There were no studies found evaluating T-VEC in patients with cutaneous lymphoma.
It is important to note that T-VEC is reserved for immunocompetent, non-pregnant patients. This therapy is contraindicated in patients with immunodeficiency and immunosuppression, such as hematologic malignancies, steroid use, active herpetic infection, antiviral therapy with acyclovir or valacyclovir, or human immunodeficiency virus/acquired immunodeficiency syndrome.2,23,24 Further, organ-transplant recipients are excluded from immunotherapy clinical trials due to the risk of graft rejection, and more data are needed for T-VEC’s utilization in this patient population.25
Additionally, it is important to consider combination therapy with T-VEC for cancer treatment. Though off-label, combining intralesional and systemic treatments has demonstrated efficacy and is likely the future of immunotherapy. In the systematic review, two trials combined T-VEC with nivolumab and two combined it with pembrolizumab for the non-melanoma cancers. However, combination therapy for advanced melanoma is an active area of research presently. T-VEC in combination with ipilimumab has demonstrated efficacy for melanoma treatment. In 2017, Chesney et al26 found that combination T-VEC and ipilimumab had a greater objective response rate (38.8%) compared to ipilimumab alone (18.0%) for melanoma patients. A follow-up randomized trial by Chesney et al27 in 2020 further demonstrated the efficacy of ipilimumab with T-VEC as therapy for advanced melanoma. The group receiving combination therapy had a 21.4 percent complete response rate compared to a 6.0 percent rate in the ipilimumab-only arm.27 Further, in the combination therapy group, they found that a CR was associated with prolonged overall survival.27 Similarly, T-VEC in combination with pembrolizumab is also a promising treatment combination for melanoma. Long et al28 found that, out of 21 melanoma patients treated with T-VEC and pembrolizumab, 14 percent had a confirmed complete response and 48 percent had a confirmed objective response rate. Comparably, a Phase Ib clinical trial by Ribas et al29 found that, out of 21 patients being treated with pembrolizumab and T-VEC combination therapy, 62 percent of patients had an objective response rate and 33 percent had a complete response rate.29 Presently, a worldwide Phase II, open-label, single-arm clinical trial is recruiting participants to evaluate combination therapy with T-VEC and pembrolizumab for advanced melanoma with an estimated primary completion date in 2021.30 Combination therapy with T-VEC is an encouraging treatment modality for melanoma, and its continued evaluation is likely to pave the way for its utility as a treatment option for other malignancies as well.
This systematic review highlights the prospective use of T-VEC as a therapy for non-melanoma cancers. Furthermore, due to the proven success of T-VEC for melanoma and the relative success reported by this review of T-VEC for MCC specifically, there is especially potential for T-VEC use as a therapy for non-melanoma skin cancers. In addition to the aforementioned University of Arizona clinical trial,20 presently, there are multiple clinical trials evaluating this utility of T-VEC. The University of Zurich is completing a Phase I study evaluating T-VEC in approximately 20 participants with locally advanced non-melanoma skin cancer with an estimated completion date of February 2022.31 Memorial Sloan Kettering Cancer center is conducting a Phase II randomized trial in approximately 19 participants evaluating T-VEC with or without radiotherapy for cutaneous melanoma, MCC, or other solid tumor cancers; the estimated completion date is June 2022.32 The National Cancer Institute is completing a Phase II study of T-VEC followed by T-VEC with nivolumab in non-melanoma skin cancers. The estimated enrollment is 68 participants and is scheduled to be completed in June 2022.33 Finally, in March 2020, Trillium Therapeutics Inc. terminated their Phase I dose-escalation trial of TTI-621 in 56 participants with relapsed and refractory solid tumors and mycosis fungoides. One arm of the study was given combination TTI-621 and T-VEC.34
The results of these trials will further increase understanding of T-VEC as a therapy for non-melanoma cancers. However, due to the relatively small total cohort of participants, further studies of non-melanoma skin cancers, such as MCC, cutaneous SCC, cutaneous lymphoma, and advanced basal cell carcinoma, among others, are warranted, with an eventual push toward Phase III trials. T-VEC is a promising new therapy, and the expansion of its clinical indication would likely be beneficial for patients.
Conclusion
Currently, T-VEC is only FDA-approved for melanoma.1 However, it has shown utility as a treatment both individually and in combination with other therapies for non-melanoma cancer and is well-tolerated by patients.8–16 These promising findings are encouraging and demonstrate the need for further Phase III trials of T-VEC.
References
- Bayan C-A, Lopez AT, Gartrell RD, et al. The role of oncolytic viruses in the treatment of melanoma. Curr Oncol Rep. 2018;20(10):60.
- Queen D, Samie FH, Zeitouni NC. How we do it: administration guide for intralesional immunotherapy with talimogene laherparepvec (T-VEC) for advanced melanoma. Dermatol Surg. 2020;46(11):1455–1457.
- Queen D, Samie FH, Zeitouni NC. Future directions and challenges facing intralesional immunotherapy with talimogene laherparepvec for advanced melanoma. Dermatol Surg. 2021;47(1):132–133.
- Perez MC, Miura JT, Naqvi SMH, et al. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma: a single-institution experience. Ann Surg Oncol. 2018;25(13):3960–3965.
- Andtbacka RHI, Laufman LH, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788.
- Louie RJ, Perez MC, Jajja MR, et al. Real-world outcomes of talimogene laherparepvec therapy: a multi-institutional experience. J Am Coll Surg. 2019;228(4):644–649.
- Kohlhapp FJ, Kaufman HL. Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. 2016;22(5):1048–1054.
- Lara KM, In GK, Matcuk Jr. GR, et al. Talimogene laherparepvec in combination with pembrolizumab leads to a complete response in a patient with refractory Merkel cell carcinoma. JAAD Case Rep. 2018;4(10):1004–1006.
- Nguyen MHK, Leong SP, Abendroth R, et al. Complete clinical response to intralesional talimogene laherparepvec injection in a patient with recurrent, regionally advanced Merkel cell carcinoma. JAAD Case Rep. 2019;5(10): 849–851.
- Westbrook BC, Norwood TG, Terry NLJ, et al. Talimogene laherparepvec induces durable response of regionally advanced Merkel cell carcinoma in 4 consecutive patients. JAAD Case Rep. 2019;5(9):782–786.
- Blackmon JT, Dhawan R, Viator TM, et al. Talimogene laherparepvec for regionally advanced Merkel cell carcinoma: a report of 2 cases. JAAD Case Rep. 2017;3(3):185–189.
- Kelly CM, Antonescu CR, Bowler T, et al. Objective response rate among patients with locally advanced or metastatic sarcoma treated with talimogene laherparepvec in combination with pembrolizumab: a phase 2 clinical trial. JAMA Oncol. 2020;6(3):402–408.
- Hu JCC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–6747.
- Miller DM, Trowbridge RM, Desai A, Drews RE. Kaposi’s varicelliform eruption in a patient with metastatic melanoma and primary cutaneous anaplastic large cell lymphoma treated with talimogene laherparepvec and nivolumab. J Immunother Cancer. 2018;6(1):122.
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16(15):4005–4015.
- Harrington KJ, Kong A, March N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26(19):5153–5161.
- Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077.
- Wang TS, Byrne PJ, Jacobs LK, et al. Merkel cell carcinoma: update and review. Semin Cutan Med Surg. 2011;30(1):48–56.
- Grabowski J, Saltzstein SL, Sadler GR, et al. A comparison of Merkel cell carcinoma and melanoma: results from the california cancer registry. Clin Med Oncol. 2008;2:327–333.
- Curial, C., A single arm phase 2 Study of talimogene laherparepvec in patients with cutaneous squamous cell cancer. 2018–2021: University of Arizona.
- Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18(10):1397–1410.
- Franklin C, Livingstone E, Roesch A, et al. Immunotherapy in melanoma: Recent advances and future directions. Eur J Surg Oncol. 2017;43(3):604–611.
- IMLYGIC (Talimogene Laherparepvec) Prescribing Information. BioVex, Inc., a subsidiary of Amgen, Inc.: Thousand Oaks, CA.
- Rehman H, Silk AW, Kane MP, et al. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53.
- Ressler J, Silmbrod R, Stepan A, et al. Talimogene laherparepvec (T-VEC) in advanced melanoma: complete response in a heart and kidney transplant patient. A case report. Br J Dermatol. 2019;181(1):186–189.
- Chesney JA, Puzanov I, Ross MI, et al. Primary results from a randomized (1:1), open-label phase II study of talimogene laherparepvec (T) and ipilimumab (I) vs I alone in unresected stage IIIB- IV melanoma. J Clin Oncol. 2017;35(15_Suppl):9509–9509.
- Chesney JA, Puzanov, Collichio FA, et al, Association between complete response and survival in advanced melanoma treated with talimogene laherparepvec (T-VEC) plus ipilimumab (ipi). J Clin Oncol. 2020;38(15_Suppl):10029–10029.
- Long GV, Dummer R, Ribas A, et al. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene laherparepvec (T-VEC) and pembrolizumab (pembro) for unresectable stage IIIB-IV melanoma. J Clin Oncol. 2016;34(15_Suppl):9568–9568.
- Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109–1119.e10.
- Chesney JA, Milhem MM, Chaney MF, et al. Design and rationale of MASTERKEY-115 phase II trial of talimogene laherparepvec (T-VEC) with pembrolizumab (pembro) in patients with advanced melanoma who progressed on prior anti-programmed cell death-1 (anti-PD-1) therapy. J Clin Oncol. 2020;38(15_Suppl).
- Dummer D, Goldinger SM. A phase I, open label, single arm, single centre study to evaluate mechanism of sction of talimogene laherparepvec (T-VEC) in locally advanced non-melanoma skin cancer. 2018–2020: University of Zurich.
- Barker C. A phase II randomized trial of intralesional talimogene laherparepvec (TALIMOGENE LAHERPAREPVEC) with or without radiotherapy for cutaneous melanoma, Merkel cell carcinoma, or other solid tumors. 2016–2021: Memorial Sloan Kettering Cancer Center.
- Silk AW. A phase II study of talimogene laherparepvec followed by talimogene laherparepvec + nivolumab in refractory T cell and NK cell lymphomas, cutaneous squamous cell carcinoma, Merkel cell carcinoma, and other rare skin tumors. 2017–2021: National Cancer Insititute.
- T.T. Inc., Editor. A phase 1 dose escalation trial of intratumoral injections of TTI-621 in subjects with relapsed and refractory percutaneously-accessible solid tumors and mycosis fungoides, 2016–2020: Trillium Therapeutics Inc.

