 by Melissa Mei-Hsia Chan, MD; Lucinda Siyun Tan, MBBS, MRCP (UK), MMed (Int. Med); Yung-Hian Leow, MBBS, MMed (Int. Med); Anthony Teik-Jin Goon, MBBS, MRCP (UK), FRCP (Edin), PhD; and Chee Leok Goh, MD, MBBS, MMed (Int. Med), MRCP (UK), FRCP (Edin)
by Melissa Mei-Hsia Chan, MD; Lucinda Siyun Tan, MBBS, MRCP (UK), MMed (Int. Med); Yung-Hian Leow, MBBS, MMed (Int. Med); Anthony Teik-Jin Goon, MBBS, MRCP (UK), FRCP (Edin), PhD; and Chee Leok Goh, MD, MBBS, MMed (Int. Med), MRCP (UK), FRCP (Edin)
Dr. Chan is with the Duke-NUS Medical School in Singapore. Drs. Tan, Leow, Goon, and Goh are with the Department of Dermatology at the National Skin Centre in Singapore.
FUNDING: This study was supported by internal research funds from the Medical Department Fund from National Skin Centre, Singapore.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. We compared the irritancy potential of sodium lauryl sulfate (SLS)-free aqueous cream to SLS-containing aqueous cream and other moisturizers.
Design: This was a double-blind, intraindividual occlusive study. SLS-containing aqueous cream; SLS-free aqueous cream; white soft paraffin; urea cream; Physiogel® (Stiefel Laboratories, Brentford, United Kingdom); QV cream (Ego Pharmaceuticals Pty. Ltd., Braeside, Australia); Cetaphil RestoraDerm® (Galderma Laboratories, Fort Worth, Texas); Ceradan® (Hyphens Pharma International Ltd., Singapore); normal saline; and SLS 1% aqueous were applied with Finn chamber occlusion to different sites on each participant’s back for 72 hours. Skin assessments were carried out on Day 0 preapplication and Day 3 and Day 7 postapplication.
Participants. Twelve healthy adult volunteers were included in this study.
Measurements. Study subjects were clinically evaluated by an experienced dermatologist using a four-point severity scale to assess the severity of erythema, dryness, desquamation, stinging or burning, and pruritus. Corneometer® and transepidermal water loss (TEWL) readings were taken to assess skin hydration and skin barrier integrity, respectively. All measurements were performed on Days 0, 3, and 7.
Results. Application of the SLS-free aqueous cream resulted in no significant changes in TEWL or Corneometer® readings throughout the study period. The SLS-containing aqueous cream resulted in a significant increase in TEWL from Day 0 to Days 3 and 7. All test moisturizer creams showed no significant changes in their clinical assessment scores.
Conclusion. The results of our study indicate that SLS-free aqueous cream has a lower irritancy potential than SLS-containing aqueous cream, with the same level of maintenance of skin barrier integrity and hydration. SLS-free aqueous cream also appears to be less irritating to the skin than other non-SLS generic and commercial moisturizers tested.
KEYWORDS: Aqueous cream, moisturizers, sodium lauryl sulfate (SLS)
J Clin Aesthet Dermatol. 2019;12(7):52–58
Aqueous cream BP (British Pharmacopoeia) has been used as a moisturizer for xerotic skin conditions since 1958.1 It is a light, paraffin-based, oil-in-water emulsion, compounded from liquid paraffin, white soft paraffin, phenoxyethanol, purified water, and emulsifying wax.1 Cetostearyl alcohol and sodium lauryl sulfate (SLS) comprise the emulsifying wax, which provides the consistency and base for the oil-water phase mixing of aqueous cream.1 However, recent reports on adverse cutaneous reactions from SLS-containing aqueous cream has attracted criticism to the previously popular moisturizer.2 This prompted the Medicines and Healthcare products Regulatory Agency to issue a Drug Safety Update in March 2013 with an official warning that aqueous cream is likely to cause skin irritation.2 Research has shown that SLS-containing aqueous cream thins the stratum corneum, increases epidermal turnover rate, provokes greater transepidermal water loss (TEWL), decreases corneocyte maturity and size, and accelerates inflammatory protease activity.3–6 Several authors have postulated that the skin irritancy effects are secondary to SLS, a known irritant, and have since recommended against the use of SLS-containing aqueous cream, both as a leave-on emollient and a wash-off product.7
Despite the recent criticism of aqueous cream, it remains one of the most commonly used, inexpensive moisturizers on the market.8–10 In addition, SLS-free aqueous cream is now widely available as a substitute for SLS-containing aqueous cream.11 To our knowledge, there has not yet been a comprehensive study to evaluate the irritancy potential of SLS-free aqueous cream. Therefore, the aim of this pilot study was to compare the irritancy potential of SLS-free aqueous cream to SLS-containing aqueous cream and other generic and commercial moisturizers. We hope that this study will aid in appraising moisturizers with the least irritation potential, ensuring safer and more affordable patient care.
Materials and Methods
This study was conducted at the National Skin Centre, a tertiary dermatologic referral center in Singapore. Institutional review board approval was obtained prior to commencement of the study (NHG DSRB reference no. 2011/01773). Informed consent was obtained from all participants.
Materials. This study assessed eight different moisturizers (Table 1). SLS 1% aqueous and normal saline served as the positive and negative controls, respectively. The moisturizers that were chosen for testing are commonly prescribed to patients with dry skin at the National Skin Centre. The SLS 1% aqueous formulation was prepared by the institution’s pharmacy and comprises 1% of SLS in deionized water.

Methods. The evaluation of the moisturizers was conducted through intramoisturizer and intermoisturizer comparisons. Eligible participants were healthy volunteers 21 years of age or older with no history of atopy or any dermatological condition at the time of study. The study participants agreed to abstain from bathing and/or applying cosmetics and skincare products over their tested skin region during the study period. Study participants were also discouraged from participating in any activity that might affect skin properties, such as sunbathing, exfoliation, or performing other beauty treatments during the study period. The exclusion criteria for our study are as follows:
- Age younger than 21 years
- History of atopy, including asthma, allergic rhinitis, or atopic dermatitis
- Current diagnosis of any dermatological condition at the time of the study
- Inability or unwillingness to leave the Finn chambers on their back and avoid bathing, applying moisturizers or cosmetics, exfoliating, and sun exposure to the test area for 72 hours
- Presence of known adverse reactions to any of the moisturizers to be tested
- Pregnancy
- Presence of excessive hair on the back
Patch test chambers were used to evaluate the skin irritancy potential of the moisturizers.12 Ten skin regions located on each participant’s back were selected as application sites and carefully outlined. Intraindividual and regional differences in the skin barrier were minimized by confining application sites to the back between the scapulae and waist, while keeping application sites in close proximity to each other without overlapping. Each subject had the moisturizers applied to different sites so that no one moisturizer was applied to the same skin area across all participants (Figure 1). After initial skin assessments on Day 0, a needleless syringe was used to deliver 1mL of each test moisturizer cream to the 12mm Finn chambers on adhesive tape (Scanpor®, Epitest Ltd. Oy, Tuusula, Finland) that were then used to occlude the treatment sites for 72 hours. Our methodology drew on the protocol of the modified Draize test for evaluating skin irritancy.13,14 As mentioned previously, participants were instructed to avoid bathing their backs and to refrain from exposing the application sites to activities or products that might have affected the skin properties throughout the study period, even after the removal of the Finn chambers from Days 3 to 7. Participants were permitted to gently clean their back with a dry, soft towel for hygiene purposes and were instructed to keep the application sites covered by wearing a shirt throughout the study period.

Assessments. Clinical assessments and biophysical measurements of the skin were performed on Day 0, prior to moisturizer applications, and on Days 3 and 7, after application of the moisturizers and the removal of the patch test chambers. Upon the removal of the Finn chambers from the application sites, each participant’s back was thoroughly and gently wiped with a dry towel to remove any residual test cream that might interfere with the assessments. Prior to skin assessments, study participants were acclimatized to an environmentally controlled room with a fixed temperature range of 20° to 22°C and relative humidity range of 40 to 60 percent over a 20-minute duration. Following similar methodologies in grading skin complications, an experienced dermatologist evaluated the degrees of erythema, dryness, desquamation, stinging or burning, and pruritus using a four-point severity scale, where 0=none and 3=severe.15
TEWL evaluates skin barrier integrity by measuring the degree of water evaporation from the skin.16 TEWL was measured on Days 0, 3, and 7 using a Tewameter® TM 300 (Courage and Khazaka Electronic GmbH, Cologne, Germany). A dry cloth was used to clean the skin region prior to the measurements. The average measurement was calculated from three TEWL readings taken from each treatment site and was used for analysis.
Skin hydration was assessed with the Corneometer CM825 (Courage and Khazaka Electronic GmbH, Cologne, Germany), which measures the dielectric capacitance of the stratum corneum.17 According to the Corneometer, the range of values that reflect skin hydration is between 0 to 130 arbitrary units (AU), with higher values correlating with increased levels of skin hydration.18 Measurements were conducted on Days 0, 3, and 7, and the average measurement calculated from three Corneometer readings of each treatment site was used for analysis.
Throughout the double-blinded study period, blinded study participants and research assistants performed moisturizer administrations and data collection, while unblinded pharmacists helped with the moisturizer storage, dispensing, and preparation. The integrity of the double-blinded study was monitored, and no premature unblinding was observed.
Statistical analysis. Patient demographics; clinical review scores for erythema, dryness, desquamation, stinging or burning, and pruritus; TEWL measurements; and Corneometer readings were summarized. The Wilcoxon sign-ranked test for matched data was used to test for the change from baseline (Day 0) for each moisturizer, comparing the results at Days 3 and 7 postapplication with baseline, with statistical significance denoted by p-value [1]. The same statistical test method was also used to test for statistically significant differences in the change from baseline between a particular moisturizer and SLS-free aqueous cream, where p-value [2] is observed. The level of significance was set at 0.05. Results were represented as medians with minimum (min) and maximum (max) ranges, or as means with standard deviations (SDs). The STATA version 14 software (StataCorp LLC, College Station, Texas) was used for the statistical analysis.
Results
Twelve healthy volunteers (5 men, 7 women) participated in this study. Their mean age was 38.3±13.2 years and they had Fitzpatrick Skin Types IV and V. No reports of adverse events occurred during the study period. Based on clinical assessments, all test moisturizer creams showed no statistically significant changes from baseline in their scores for erythema, dryness, desquamation, stinging or burning, and pruritus throughout the study period on Days 3 and 7. An increase in TEWL on Day 3 from baseline presentation was observed on skin sites occluded with SLS-containing aqueous cream, as seen in Table 2, showing a median (range) increase of 2.9g/m2/h (-11.7–16.8)(p-value [1]=0.02). Similarly, our positive control of SLS 1% aqueous showed a significant median (range) increase in TEWL of 3.8g/m2/h (1.4–9.5) (p-value [1]<0.01) on Day 3. However, SLS-free aqueous cream did not cause any significant change in TEWL readings from baseline at Day 3 (p-value [1]=0.06) or Day 7 (p-value [1]=0.86).
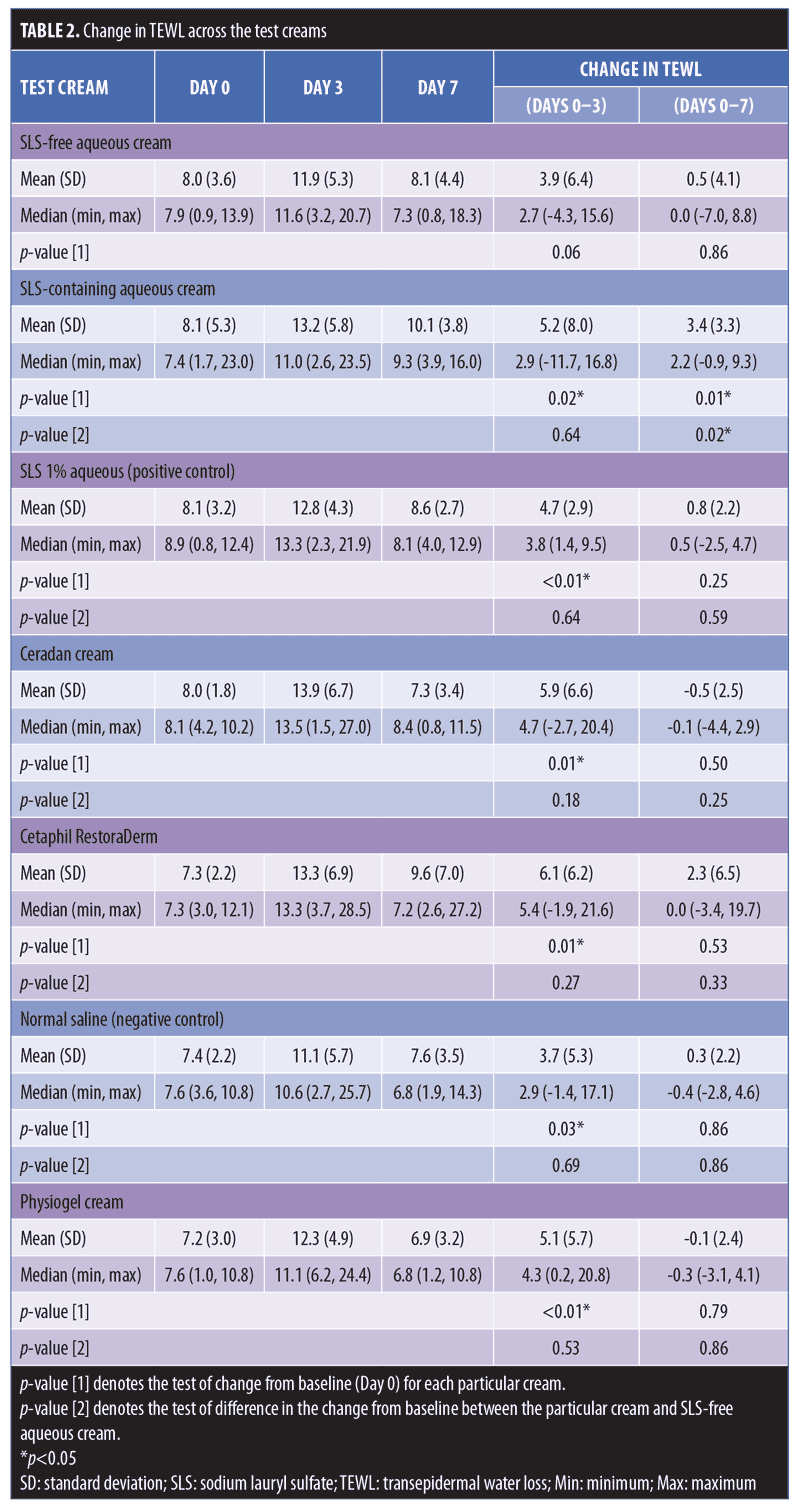
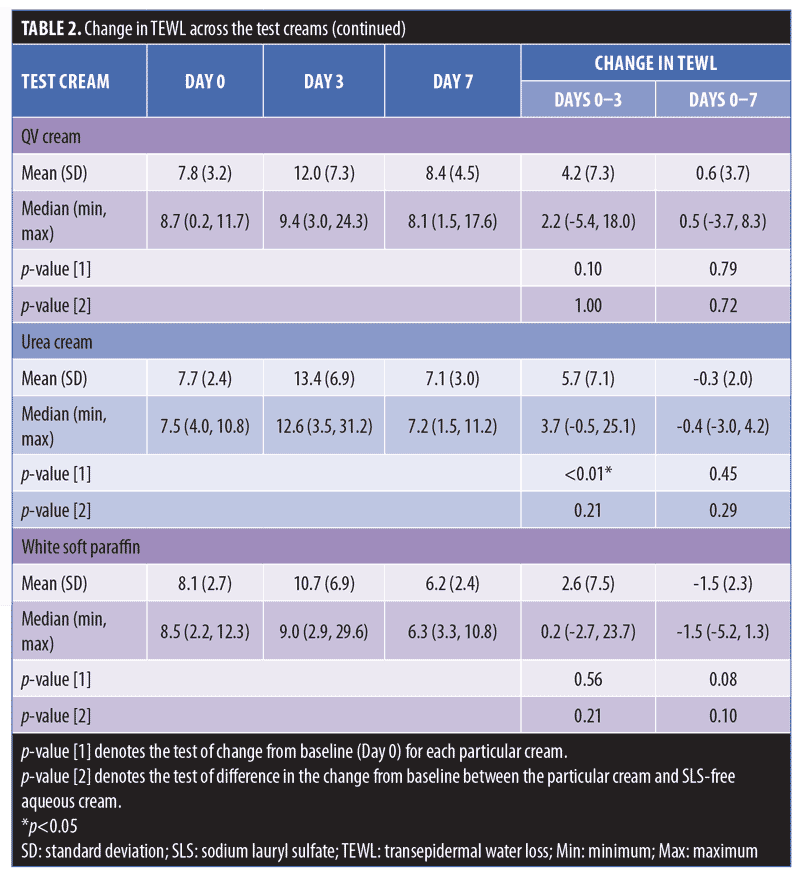
Physiogel® (Stiefel Laboratories, Brentford, United Kingdom; p-value [1]<0.01; Cetaphil RestoraDerm® (Galderma Laboratories, Fort Worth, Texas) p-value [1]=0.01; Ceradan® (Hyphens Pharma International Ltd., Singapore) p-value [1]=0.01; and urea cream p-value [1]<0.01 showed significant median increases in TEWL from baseline to Day 3. However, there were no significant changes in TEWL values from baseline to Day 7, reflecting a recovery of skin barrier integrity. In contrast, SLS-containing aqueous cream sites showed a slower recovery of skin barrier integrity compared to non-SLS moisturizer sites, with a significant median (range) increase in TEWL of 2.2g/m2/h (-0.9–9.3) (p-value [1]=0.01), which continued to be observed at Day 7, compared to baseline. Figures 2 and 3 summarize these findings graphically.

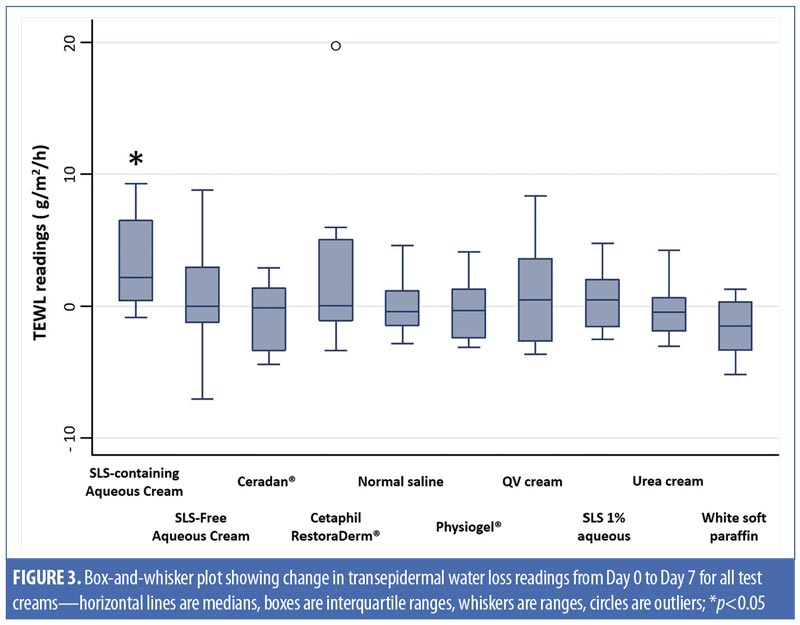
In addition, intermoisturizer comparisons showed a statistically significant difference in the change of TEWL from baseline to Day 7 between SLS-free aqueous cream and SLS-containing aqueous cream (p-value [2]=0.02), as seen in Table 2. Other moisturizers, however, showed no significant difference in the change of TEWL from baseline to Day 3 and Day 7 compared to SLS-free aqueous cream.
Table 3 compares the skin hydration of SLS-containing aqueous cream (p-value [1]=0.88) and SLS-free aqueous cream (p-value [1]=0.81). There was no statistically significant decrease in Corneometer readings from baseline to Day 3. On the contrary, we observed a decrease in the hydration on skin occluded with SLS 1% aqueous (positive control), with a significant median (range) decrease in Corneometer reading of -12.1AU (-25.1–18.4) (p-value [1]=0.02) from baseline to Day 3.
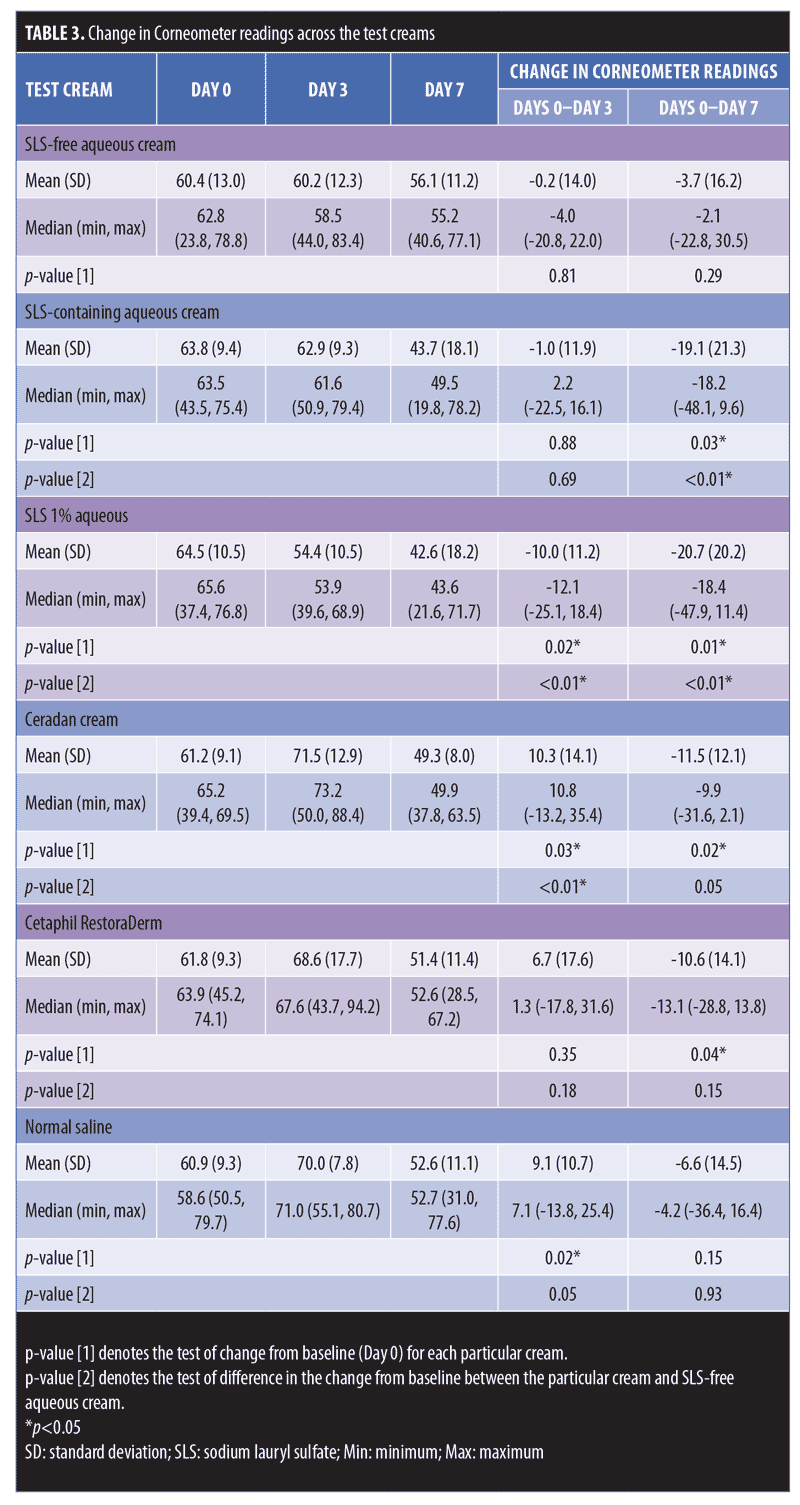
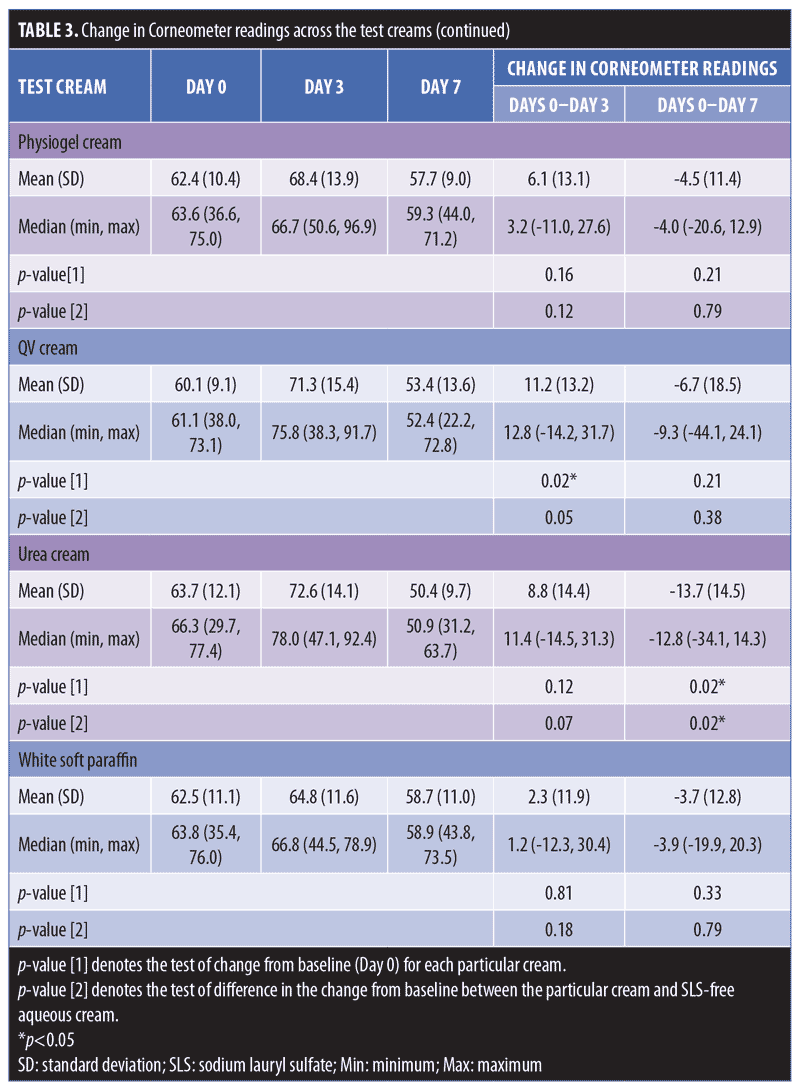
When assessing skin hydration from baseline to Day 7, both SLS-containing aqueous cream and positive control SLS 1% aqueous showed similar statistically significant decreases in corneometer readings, with a median (range) decrease of -18.2AU (-48.1–9.6) (p-value [1]=0.03) and -18.4AU (-47.9–11.4) (p-value [1]=0.01), respectively. On the contrary, SLS-free aqueous cream showed no significant change in corneometer reading from baseline to Day 7 (p-value [1]=0.29).
Aside from SLS-containing aqueous cream and SLS 1% aqueous, other moisturizers that showed significant decreases in Corneometer readings from baseline to Day 7 include Cetaphil RestoraDerm, Ceradan, and urea cream (p-values [1]=0.04, 0.02, and 0.02, respectively). Skin hydration was maintained for Physiogel, QV cream, and white soft paraffin throughout the study period. Figure 4 summarizes these findings graphically.
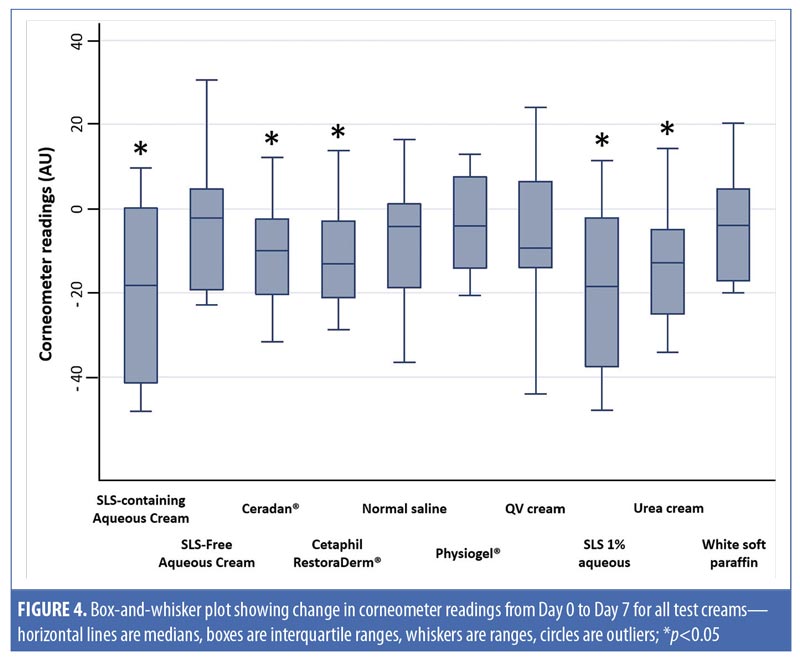
Table 3 also shows intermoisturizer comparisons wherein a statistically significant difference in the change of corneometer readings from baseline to Day 7 is observed between SLS-free aqueous cream and SLS-containing aqueous cream (p-value [2] <0.01). Aside from urea cream (p-value [2]=0.02), all other moisturizers showed no significant difference in the change of Corneometer readings from baseline to Day 7 when compared to SLS-free aqueous cream.
Discussion
In our study, both SLS-containing aqueous cream and positive control SLS 1% aqueous caused a significant increase in TEWL readings from baseline to Day 3. This finding was also observed with Cetaphil RestoraDerm, Ceradan, Physiogel, and urea cream. However, unlike SLS-containing aqueous cream, all other moisturizers demonstrated skin barrier recovery by Day 7, with no significant increase in TEWL from baseline to Day 7. Cetaphil RestoraDerm, Ceradan, Physiogel, and urea cream appeared to cause mild disruption to the skin barrier when applied to the skin. Notably, skin sites treated with SLS-free aqueous cream showed no significant increase in TEWL from baseline to Day 3 and from baseline to Day 7, suggesting that SLS-free aqueous cream did not damage skin barrier integrity throughout the occlusion and postocclusion periods. This is in contrast with the skin irritation caused by SLS-containing aqueous cream, Cetaphil RestoraDerm, Ceradan, and Physiogel.
Our findings of TEWL from baseline to Day 7 across the moisturizers suggests that SLS in SLS-containing aqueous cream contributes to the disruption of skin barrier integrity that increases TEWL, and that SLS-containing aqueous cream appears to cause more barrier function damage and likely delays recovery of skin barrier integrity compared to other moisturizers. The positive control of SLS 1% aqueous showed a recovery of skin barrier integrity by Day 7, suggesting that the slower recovery seen with SLS-containing aqueous cream might not be entirely due to the effect of SLS, but might also be caused by potential irritants present in the aqueous cream formulation.8
Our study also showed that SLS-containing aqueous cream, Cetaphil RestoraDerm, Ceradan, and urea cream caused skin barrier dysfunction, leading to reduced skin hydration as reflected by the significant decreases in Corneometer readings from baseline to Day 7. SLS-free aqueous cream appeared to provoke no significant changes in Corneometer readings throughout the study period. These findings suggest that SLS-free aqueous cream is not irritating to the skin and maintains skin barrier integrity, unlike SLS-containing aqueous cream. In addition, SLS-free aqueous cream appears to maintain skin hydration better than Cetaphil RestoraDerm and Ceradan.
The skin irritancy effects of SLS that were observed in our study are in concordance with earlier published research.3–7,18 SLS disrupts the normal skin barrier by inducing the swelling of corneocytes, the denaturation of keratin, and by increasing skin surface pH.4 This disruption leads to a loss of skin integrity and dehydration, resulting in increased TEWL and decreased corneometer readings, respectively. SLS that causes skin barrier disruption would allow further intraepidermal SLS penetration and accelerate skin barrier disruption with the release of more proinflammatory cytokines.19,20
While we previously suggested that SLS might not be the only constituent in SLS-containing aqueous cream responsible for skin barrier disruption, our findings suggest that SLS-free aqueous cream is safe and nonirritating to the skin, with no barrier disruption, as evidenced by the maintenance of normal TEWL values throughout the study period. As such, we postulate that SLS might not only increase skin permeability, but also enhance other skin irritants present in SLS-containing aqueous cream that would further exacerbate skin irritation. This hypothesis is supported by other studies that show the usage of SLS as a penetration enhancer in transdermal drug delivery.21–23
We acknowledge the cost advantages of SLS-free aqueous cream compared to other commercial moisturizers. Not only did SLS-free aqueous cream appear to conserve the natural skin barrier integrity and maintain skin hydration, but it also did not cause obvious skin irritation, based on the observed absence of skin erythema, dryness, desquamation, stinging or burning, and/or pruritus. Our study demonstrates that SLS-free aqueous cream has a lower irritancy potential than SLS-containing aqueous cream and some commercial moisturizers.
Limitations. We acknowledge that several limitations exist in our pilot study. First, our small sample size of 12 study participants leads to a lower statistical power and estimated confidence compared to other, larger studies. In addition, we employed the method of single occlusive patch testing on study participants to assess the moisturizers. Although easy and quick to perform, patch testing does not accurately mimic real-life circumstances, as opposed to repeated open application testing.24,25 Moreover, using the skin on the backs of participants might not be a fair representation of the general skin. However, we recognize that the skin on the back is the area with the least exposure to environmental conditions which might have confounded our results. Using the back was also the most convenient test location for our study participants.
Conclusion
With its low irritancy potential and ability to maintain skin integrity and hydration, SLS-free aqueous cream appears to be a safe and effective alternative to more expensive commercial moisturizers. Nevertheless, a larger study is needed to better characterize the response to various moisturizers and thereby enable us to individualize our approach in prescribing moisturizers for patients. We hope our pilot and hypothesis-generated study will serve as a primer for future research into SLS-free aqueous cream as a safe and cost-effective moisturizer.
References
- British Pharmacopoeia. London, England: The Pharmaceutical Press; 1958.
- Medicines and Healthcare products Regulatory Agency. Drug safety update: aqueous cream: may cause skin irritation, particularly in children with eczema, possibly due to sodium lauryl sulfate. Available at: https://www.gov.uk/drug-safety-update/aqueous-cream-may-cause-skin-irritation. Accessed April 16, 2018.
- Mohammed D, Matts PJ, Hadgraft J, Lane ME. Influence of Aqueous Cream BP on corneocyte size, maturity, skin protease activity, protein content and transepidermal water loss. Br J Dermatol. 2011;164(6):1304–1310.
- Cork MJ, Danby S. Aqueous cream damages the skin barrier. Br J Dermatol. 2011;164(6): 1179–1180.
- Tsang M, Guy RH. Effect of Aqueous Cream BP on human stratum corneum in vivo. Br J Dermatol. 2010;163(5):954–958.
- Danby SG, Al Enezi T, Sultan A, et al. The effect of aqueous cream BP on the skin barrier in volunteers with a previous history of atopic dermatitis. Br J Dermatol. 2011;165(2):329–334.
- Moncrieff G, Cork M, Lawton S, et al. Use of emollients in dry skin conditions: consensus statement. Clin Exp Dermatol. 2013;38(3): 231–238.
- Verbov J. The aqueous cream controversy. Clin Exp Dermatol. 2014;39(1):92–93.
- Mack Correa MC, Nebus J. Management of patients with atopic dermatitis: the role of emollient therapy. Dermatol Res Pract. 2012;2012:836931.
- Hon KL, Leung AK. Use of ceramides and related products for childhood-onset eczema. Recent Pat Inflamm Allergy Drug Discov. 2013;7(1):12–19.
- Trueman E. Managing radiotherapy induced skin reactions in the community. J Comm Nurs. 2013;27(4):16–24.
- Johansen JD, Aalto-Korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—recommendations on best practice. Contact Dermatitis. 2015;73(4):195–221.
- Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82(3):377–390.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 6th ed. Raleigh, NC: PMPH-USA; 2008: 30–34.
- Goh CL, Tang MB, Briantais P, et al. Adapalene gel 0.1% is better tolerated than tretinoin gel 0.025% among healthy volunteers of various ethnic origins. J Dermatolog Treat. 2009;20(5):282–288.
- Darlenski R, Sassning S, Tsankov N, Fluhr JW. Non-invasive in vivo methods for investigation of the skin barrier physical properties. Eur J Pharm Biopharm. 2009;72(2):295–303.
- Constantin MM, Poenaru E, Poenaru C, Constantin T. Skin hydration assessment through modern non-invasive bioengin-eering technologies. Maedica (Buchar). 2014;9(1):33–38.
- Danby SG, Chalmers J, Brown K, et al. A functional mechanistic study of the effect of emollients on the structure and function of the skin barrier. Br J Dermatol. 2016;175(5):1011–1019.
- De Jongh CM, Verberk MM, Withagen CE, et al. Stratum corneum cytokines and skin irritation response to sodium lauryl sulfate. Contact Dermatitis. 2006;54(6):325–333.
- De Jongh CM, Jakasa I, Verberk MM, Kezic S. Variation in barrier impairment and inflammation of human skin as determined by sodium lauryl sulfate penetration rate. Br J Dermatol. 2006;154(4):651–657.
- Borrás-Blasco J, Lopez A, Morant MJ, et al. Influence of sodium lauryl sulfate on the in vitro percutaneous absorption of compounds with different lipophilicity. Eur J Pharm Sci. 1997;5(1):15–22.
- Yamato K, Takahashi Y, Akiyama H, et al. Effect of penetration enhancers on transdermal delivery of propofol. Biol Pharm Bull. 2009;32(4):677–683.
- Som I, Bhatia K, Yasir M. Status of surfactants as penetration enhancers in transdermal drug delivery. J Pharm Bioallied Sci. 2012;4(1):2.
- Villarama CD, Maibach HI. Correlations of patch test reactivity and the repeated open application test (ROAT)/provocative use test (PUT). Food Chem Toxicol. 2004;42(11):1719–1725.
- Geier J, Uter W, Pirker C, Frosch PJ. Patch testing with the irritant sodium lauryl sulfate (SLS) is useful in interpreting weak reactions to contact allergens as allergic or irritant. Contact Dermatitis. 2003;48(2):99–107.

