 J Clin Aesthet Dermatol. 2021;14(3):E53–E57.
J Clin Aesthet Dermatol. 2021;14(3):E53–E57.
by Terry M. Jones, MD, and Iain Stuart, PhD
Dr. Jones is with J&S Studies Inc., in College Station, Texas. Dr. Stuart is with VYNE Therapeutics Inc., in Bridgewater, New Jersey.
FUNDING: This study was sponsored by Foamix Pharmaceuticals Ltd., a wholly owned subsidiary of VYNE Therapeutics Inc.
DISCLOSURES: Dr. Jones served as the principal investigator on the study. Dr. Stuart is an employee and stockholder at VYNE Therapeutics Inc.
ABSTRACT: Background: FMX103 1.5% is the first and only topical minocycline foam that is approved for the treatment of papulopustular rosacea in adults.
Objective: We sought to characterize the safety and pharmacokinetics of minocycline under maximal-use conditions of FMX103 1.5% in subjects with moderate-to-severe rosacea.
Methods: This Phase I, single-center, nonrandomized, open-label, single-period pharmacokinetics and safety evaluation study evaluated multiple-dose, topical administration of FMX103 1.5%. Twenty subjects meeting study inclusion/exclusion criteria had ~2 grams of FMX103 1.5% applied to the full face once per day for 14 days. Blood samples were collected 30 minutes prior to study drug application on treatment Days 1, 2, 6, 9, 11, 12, and 14, and also at 2, 4, 8, 12, 16, and 24 hours post-administration on treatment Days 1 and 14.
Results: Following topical application of a 2-gram maximal-use dose of FMX103 1.5% for 14 days, minocycline plasma concentrations were low. Overall, trough levels were approximately 0.5ng/mL from 24 hours after the first dose through 24 hours after the last dose on Day 14, indicating that steady-state levels appear to have been reached within the first day of dosing. Daily application of FMX103 1.5% was generally safe and well-tolerated by all subjects.
Conclusion: Once-daily topical application of FMX103 1.5% did not lead to appreciable systemic exposure or accumulation of minocycline, suggesting that it is a viable treatment option for papulopustular rosacea.
Keywords: Minocycline, open-label, papulopustular rosacea, pharmacokinetics, Phase I study, maximum use, topical foam
Papulopustular rosacea is a chronic dermatologic condition that predominantly affects the face.1 In the United States, the estimated prevalence of rosacea of all types ranges from 1 to 5 percent, with as many as 16 million people being affected.1,2 Caucasians with fair, sun-sensitive skin, particularly those of Celtic or Northern European descent, appear to have the greatest risk for rosacea, and women are more commonly affected than men.1,3
Rosacea is a syndrome of undetermined etiology characterized by both vascular and papulopustular components predominantly involving the face and occasionally the neck and upper trunk.1,4 Clinical findings include mid-facial erythema, telangiectasia, papules and pustules, and sebaceous gland hypertrophy.1,3,4 Secondary symptoms may include itching, stinging, and burning, along with patients’ complaints of increased sensitivity of the facial skin.3,5 The cosmetic disfigurement associated with rosacea has a strong psychosocial impact that can negatively affects a patient’s emotional health and quality of life.3,6
Numerous triggers can precipitate the clinical manifestations of rosacea, including exposure to ultraviolet light, extremes of hot or cold temperatures, and the consumption of alcohol, hot drinks, and/or spicy foods.1,5 Patients are generally advised to avoid known triggers or provocation factors.1,5,7
First-line treatment for inflammatory papules/pustules of rosacea often initially involves a combination of light devices, topical, and/or oral therapy, followed by a single therapy to maintain remission.8 Topical therapies approved by the United States Food and Drug Administration (FDA) for inflammatory papules/pustules of rosacea include 15% azelaic acid, 1% metronidazole, 1% ivermectin cream, and 10% sodium sulfacetamide. Oral therapy includes an FDA-approved modified-release doxycycline involving a subantimicrobial dose, off-label oral antibiotics (including tetracycline, minocycline, and doxycycline), and oral isotretinoin.8 Topical treatments are often used as initial therapies for mild or moderate rosacea, while systemic oral therapies are often utilized as monotherapy or in combination with topicals in moderate-to-severe cases.1,7,9 Oral tetracyclines, such as doxycycline and minocycline, in particular have been the predominant class of systemic medications used in rosacea therapy.7,9,10 However, these agents have been associated with systemic adverse effects involving the gastrointestinal tract and other body systems. Such effects from oral antibiotics may be circumvented by topical therapy.
FMX103 1.5% is a novel, topical foam formulation of minocycline that was developed to facilitate improved local delivery of minocycline to affected skin while limiting systemic exposure and associated systemic adverse events. The safety and efficacy of FMX103 1.5% for the treatment of papulopustular rosacea have been demonstrated in one Phase II11 and two Phase III12 randomized, double-blind, vehicle-controlled studies. Subjects who were treated with FMX103 1.5% for 12 weeks exhibited significantly greater reductions in inflammatory lesions and significantly greater improvements in Investigator’s Global Assessment (IGA) scores than patients who received vehicle, starting as early as four weeks into treatment.11,12 In these studies, FMX103 1.5% was found to be generally safe and well-tolerated.11,12 Equally, a recent open-label study demonstrated that the favorable safety profile of FMX103 1.5% was maintained over an extended treatment period lasting for up to one year.13
It is important to understand the level of systemic exposure to minocycline from FMX103 1.5% use at dosing multiples significantly in excess of the expected mean daily product consumption to better characterize the safety profile of this novel topical treatment. In this regard, the purpose of this Phase I open-label study was to evaluate the safety and pharmacokinetic (PK) profile of minocycline under maximum-use treatment conditions during 14 days of once-daily dosing in subjects with moderate-to-severe facial papulopustular rosacea.
Methods
Study design. This was an open-label study designed to characterize minocycline PK and evaluate the safety of FMX103 1.5% after once-daily administration for 14 days (Figure 1) under maximum-use conditions in healthy subjects, aged 18 years or older, with moderate-to-severe facial papulopustular rosacea. Eligible subjects included nonsmoking male subjects and nonpregnant female subjects with a history of erythema and/or flushing on the face who agreed to minimize their exposure to external rosacea triggers during the course of the study. Subjects with any skin condition or excessive facial hair that would interfere with the assessment of rosacea were excluded from the study, as were subjects with a history of hypersensitivity or allergy to minocycline or those with a history of ocular rosacea. Subjects were also excluded if, within 1 to 6 months of screening (dependent on the type of therapy), they had received oral or topical retinoids, systemic antibiotics or corticosteroids, or any substance known to be a strong cytochrome P450 inhibitor or had initiated use of oral estrogens or contraceptives.
Approximately 2 grams of FMX103 1.5% were applied topically to the full face, regardless of whether the area was affected by rosacea, once daily from Days 1 to 14 (Figure 1). The study drug was administered in the clinic by clinic personnel at approximately the same time each day; however, on some days, subjects were permitted, with the permission of the investigator, to apply the study drug at home. On the day prior to initial dosing, subjects checked into the clinic, where they were domiciled until approximately 24 hours after study drug administration on Day 2. On Day 1, blood samples for PK analysis were obtained 30 minutes prior to study drug application, and then again at 2, 4, 8, 12, 16, and 24 (Day 2) hours post-application. On Days 6, 9, 11, and 12, blood samples were obtained 30 minutes prior to application of FMX103 1.5%, and subjects were permitted to leave the clinic one hour after study drug application. Subjects checked into the clinic again on the evening of Day 13, where they were domiciled until Day 15 after completing all final visit assessments and procedures. On Day 14, blood samples were collected 30 minutes prior to study drug application, and then again at 2, 4, 8, 12, 16, and 24 (Day 15) hours post-application. Additional blood samples for PK analysis were obtained at approximately 24-hour intervals after the Day 14 final study drug application on the mornings of Days 16, 17, and 18.
This study was conducted in accordance with the principles of the Declaration of Helsinki, in compliance with the approved protocol, Good Clinical Practice, and applicable regulatory requirements. All subjects provided written informed consent prior to their participation.
Pharmacokinetics and safety measurements. For each subject, 6mL of blood was collected at each of the 22 time points (~132 mL total) for determination of plasma minocycline concentration. Minocycline concentrations were determined using chromatographic separation on a C8 column using gradient conditions with liquid chromatography with tandem mass spectrometry (LC-MS/MS) detection according to validated methodology. The lower limit of quantification (LLOQ) was 0.257ng/mL, and any value below the limit of quantification (BLQ) was set to 0 for the purposes of PK parameters calculation. Plasma concentration data for minocycline from Day 1 and Day 14 were used to calculate PK parameters using noncompartmental methods. The plasma concentration values were summarized by sampling time point for all subjects using descriptive statistics. Determination of steady state was performed by comparison of plasma levels on Day 1, 24 hours postdose; on Days 6, 9, 11, 12, and 14, predose; and on Day 14, 24 hours postdose.
Safety was evaluated based on reports of treatment-emergent adverse events (TEAEs), results for clinical laboratory tests, physical examinations and vital signs. Adverse events (AEs) were recorded if they were reported spontaneously by the subject or observed by the investigator. Scores for rosacea severity were based on the IGA scale (5-point scale ranging from 0=clear to 4=severe).
Statistical analysis. No formal statistical determination of sample size was performed. A sample size of 20 subjects was considered sufficient to characterize the PK characteristics of topically applied minocycline. Two analysis populations were defined: 1) the all-treated population (all subjects who used the study drug at least once) and; 2) the PK population (all subjects who completed the study and had any PK data on Days 1–2, 6, 9, 11, 12, or 14–18). All summaries except summaries of PK data were based on the all-treated population.
Results
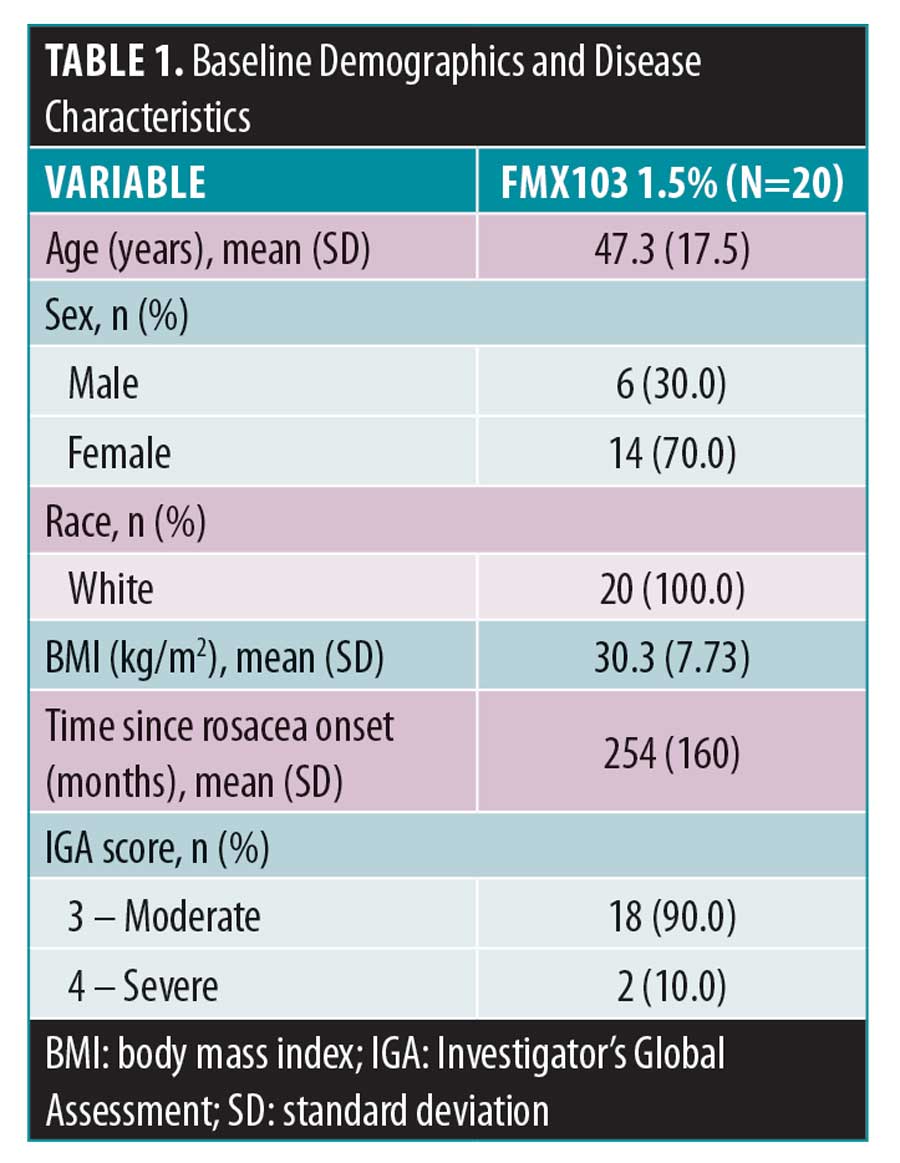
Baseline demographics and disease characteristics. In total, 20 subjects were enrolled in and completed the study. All 20 subjects were included in the all-treated and PK populations; both analysis populations were identical. Overall, subjects were 47.3 years of age, all were White (100.0%), and the majority were female (70.0%) (Table 1). At baseline, the majority of subjects (90.0%) had a rosacea severity score by IGA of 3 (moderate) and 2 (10.0%) subjects had a score of severe (IGA=4); the mean time since rosacea onset was approximately 21 years (254 months).
Extent of exposure. All 20 subjects received a single, approximately 2-gram topical application of FMX103 1.5% per day for 14 consecutive days, for a total of 14 applications. The mean (SD) total dose applied to subjects was 27.94 (0.114) grams (range: 27.7 to 28.1 grams).
Pharmacokinetic evaluation. Minocycline was quantifiable in the plasma of all subjects except for one where all minocycline plasma concentration measurements were BLQ. The reason for the lack of detectable levels of minocycline in this subject’s samples was not determined. This subject was included in the descriptive statistics of plasma minocycline concentrations; however, no PK parameters could be calculated. Another subject had a measurable concentration (0.909 ng/mL) before dosing on Day 1, but the source of this minocycline was not determined, and the low level was not considered to have an effect on PK assessments.
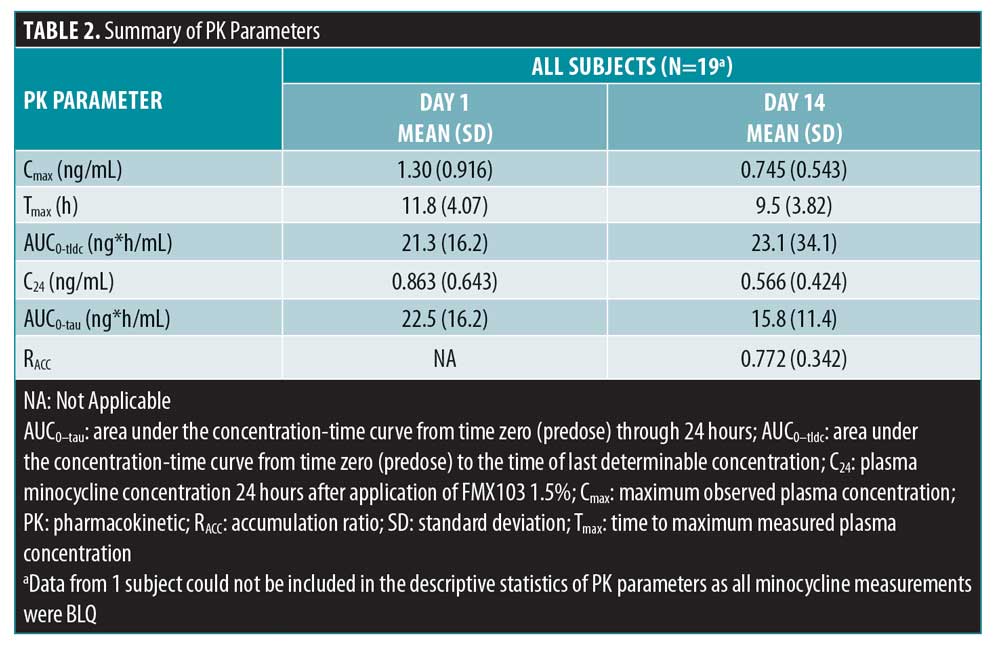
Plasma PK parameters of minocycline are presented in Table 2. The terminal-phase rate constant and T1/2 were not summarized for the PK population as these parameters could only be determined for one subject. PK parameters of minocycline were generally similar after daily application of FMX103 1.5% on Days 1 and 14 (Table 2). The mean values for the maximum observed plasma concentration (Cmax) were 1.30ng/mL and 0.75ng/mL on Days 1 and 14, respectively (Table 2). The mean AUC0-tau was approximately 22.5ng*h/mL on Day 1 and 15.8ng*h/mL on Day 14 (Table 2). Once-daily dosing of FMX103 1.5% was associated with a mean accumulation ratio of 0.772 on Day 14 (Table 2).
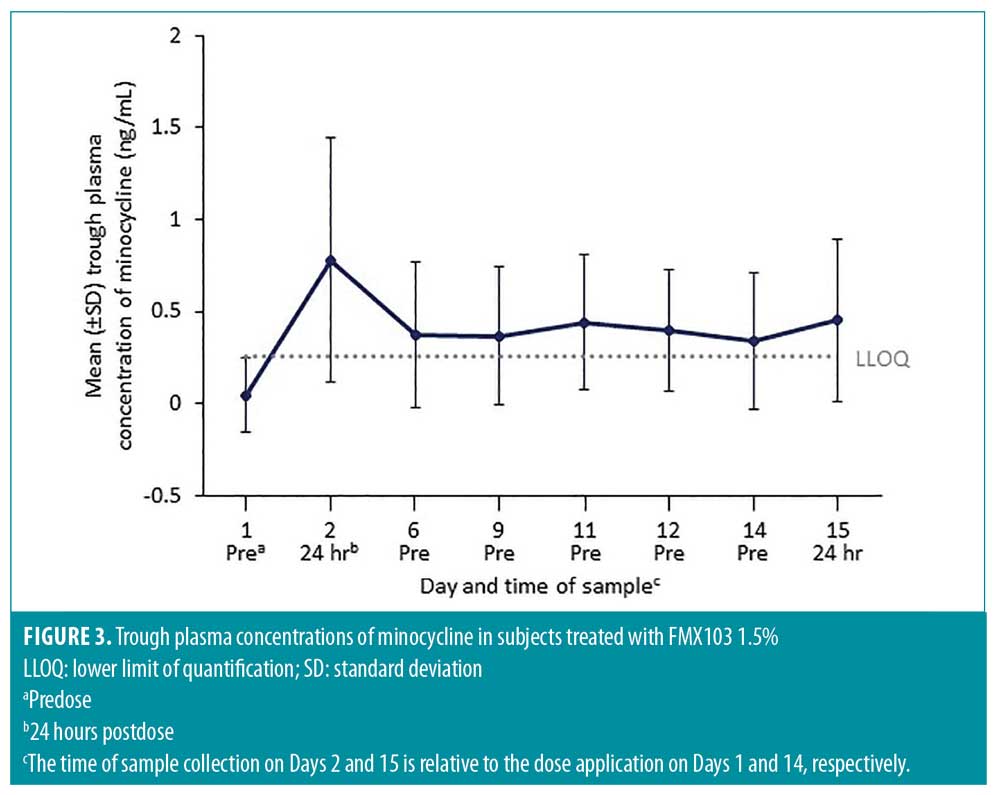
Following topical administration of the maximum-use dose (2 grams) of FMX103 1.5%, minocycline plasma concentration increased until 8 to 12 hours on Days 1 and 14 (Figure 2). Plasma concentrations of minocycline were low throughout the treatment course and remained consistent with minocycline pharmacokinetics obtained after a single 2-gram dose of FMX103 1.5% (Figure 2). Trough levels were approximately 0.5ng/mL overall, from 24 hours after the first dose through 24 hours after the Day 14 dose, with mean (standard deviation [SD]) values ranging from 0.342 (0.370) ng/mL to 0.777 (0.664), indicating that steady state appeared to be reached within one day (Figure 3).

Safety assessments. AEs are summarized in Table 3. One subject (5.0%) reported a total of two TEAEs (moderate arthralgia and mild headache). No deaths occurred during the study. There were no serious TEAEs, severe TEAEs, or TEAEs that resulted in withdrawal of study drug or dose reduction during the study. One TEAE (headache) was considered possibly related to study drug.

Changes in individual subjects from baseline to post-baseline were analyzed for hematology and chemistry parameters. There were no clinically significant findings in any subject. No TEAEs associated with laboratory abnormalities or vital signs were reported. No new or worsened abnormalities since screening were reported at the Day 13 physical examination for any subject.
Five subjects had an IGA score on Day 13 that was different from their baseline score: two subjects had a score of “severe” at baseline that decreased to “moderate” on Day 13, and three subjects had a score of “moderate” at baseline that decreased to “mild” (IGA=2) on Day 13.
Discussion
This Phase I study evaluated the safety and pharmacokinetics of minocycline in a multiple-dose, once-daily topical administration of FMX103 1.5% under maximum use treatment conditions. The PK results demonstrated minimal systemic absorption of minocycline following approximately 2 grams once-daily application of FMX103 1.5% for 14 days. Overall, minocycline levels were low across the study, with steady-state levels being achieved within the first day of application of FMX103 1.5%.
There was no evidence of accumulation of minocycline over the 14 days of treatment. Minocycline levels prior to the first application of FMX103 1.5% were detectable in one subject; however, the source of this was undetermined and considered to have no impact on the interpretation of results.
Overall, daily application of approximately 2 grams of FMX103 1.5% for 14 days was safe and well tolerated by these study subjects with facial papulopustular rosacea. There were only two TEAEs reported from one subject; neither TEAE was considered serious or severe or led to study discontinuation. Oral tetracycline-class antibiotics are known to cause hyperpigmentation in many organs, including the skin, nails, and oral cavity. The most common adverse effects of oral minocycline include fatigue, dizziness, and itch.14 These systemic effects were not seen in this study of topical minocycline foam. There were also no reported cases of autoimmune conditions, such as drug-induced lupus-like syndrome, nor of skin and hypersensitivity reactions, as have been associated with oral minocycline.14 Additionally, there were no findings of clinically significant abnormalities of laboratory values or vital signs in any subjects, and rosacea symptoms did not worsen after 14 days of treatment with FMX103 1.5%.
Conclusion
Overall, the PK results of the Phase I study showed that once-daily topical application of FMX103 1.5% did not lead to appreciable systemic exposure to minocycline. These results are consistent with the favorable safety profile that has previously been demonstrated in Phase II and Phase III trials with FMX103 1.5%. Thus, this study provides further support for the use of FMX103 1.5% as a valuable, topical treatment option for patients with rosacea.
Acknowledgments
Scient Healthcare Communications provided medical writing, editing, and publication assistance.
References
- Oge LK, Muncie HL, Phillips-Savoy AR. Rosacea: diagnosis and treatment. Am Fam Physician. 2015;92(3):187–196.
- Li WQ, Cho E, Khalili H, et al. Rosacea, use of tetracycline, and risk of incident inflammatory bowel disease in women. Clin Gastroenterol Hepatol. 2016;14(2):220-225 e221–223.
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9(1):e1361574.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92(5):234–240.
- Schaller M, Schofer H, Homey B, et al. Rosacea Management: Update on general measures and topical treatment options. J Dtsch Dermatol Ges. 2016;14 Suppl 6:17–27.
- Huynh TT. Burden of Disease: The psychosocial impact of rosacea on a patient’s quality of life. Am Health Drug Benefits. 2013;6(6): 348–354.
- Goldgar C, Keahey DJ, Houchins J. Treatment options for acne rosacea. Am Fam Physician. 2009;80(5):461–468.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: The 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82(6):1501–1510.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 5: a guide on the management of rosacea. Cutis. 2014;93(3):134–138.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 3: a status report on systemic therapies. Cutis. 2014;93(1):18–28.
- Mrowietz U, Kedem TH, Keynan R, et al. A Phase II, randomized, double-blind clinical study evaluating the safety, tolerability, and efficacy of a topical minocycline foam, FMX103, for the treatment of facial papulopustular rosacea. Am J Clin Dermatol. 2018;19(3):427–436.
- Stein Gold L, Del Rosso JQ, Kircik L, et al. Minocycline 1.5% foam for the topical treatment of moderate to severe papulopustular rosacea: Results of 2 phase 3, randomized, clinical trials. J Am Acad Dermatol. 2020;82(5):1166–1173.
- Stein Gold L, Del Rosso J, Kircik L, et al. Open-label extension study evaluating long-term safety and efficacy of FMX103 1.5% minocycline topical foam for the treatment of moderate-to-severe papulopustular rosacea. J Clin Asth Dermatol. 2020;13(11).
- Solodyn [package insert]. Scottsdale, AZ: Medicis; October 2013.

