 J Clin Aesthet Dermatol. 2023;16(5):47-49.
J Clin Aesthet Dermatol. 2023;16(5):47-49.
by Gabriella Vasile, DO; Claudia Green, BS; Hamza Bhatti, DO; Muneeb Ilyas, DO; Christopher Buckley, DO; Eli Saleeby, MD; and Eduardo Weiss, MD
Dr. Vasile is with Goodman Dermatology in Roswell, Georgia, and Philadelphia College of Osteopathic Medicine in Philadelphia, Pennsylvania. Ms. Green is a medical student in Miami, Florida. Drs. Bhatti and Saleeby are with Skin and Cancer Associates in Coral Springs, Florida. Dr. Ilyas is with Larkin Community Hospital Palm Springs in Hialeah, Florida. Dr. Buckley is with the Philadelphia College of Osteopathic Medicine in Philadelphia, Pennsylvania, and Goodman Dermatology in Roswell, Georgia. Dr. Weiss is with Hollywood Dermatology and Dermatologic Surgery at the University of Miami in Miami, Florida.
FUNDING: No funding was provided for this article
DISCLOSURES: Dr. Eduardo Weiss is a speaker for Evolus. All remaining authors have no conflicts of interest to disclose.
ABSTRACT: Background. Both onabotulinumtoxinA and prabotulinumtoxinA-xvfs are FDA-approved formulations of botulinum toxin A for the treatment of glabella and forehead rhytids.
Objective. We sought to compare the onset to action and patient satisfaction of onabotulinumtoxinA and prabotulinumtoxinA-xvfs in treating dynamic rhytids of the forehead and glabella.
Methods. Fifteen patients, aged 28 to 74, were enrolled and completed the study. Patients were randomly assigned to receive equal amounts of onabotulinumtoxinA and prabotulinumtoxinA-xvfs injected to opposite sides of the face in the glabella and forehead at Day 0 by a blinded injector. Glabellar and frontalis muscle onset to action and rhytid appearance were blindly evaluated using photographs at Days 0, 2, 4, 6, 8, 10 post-injection. Patients rated their satisfaction of left and right sides using a standardized scale.
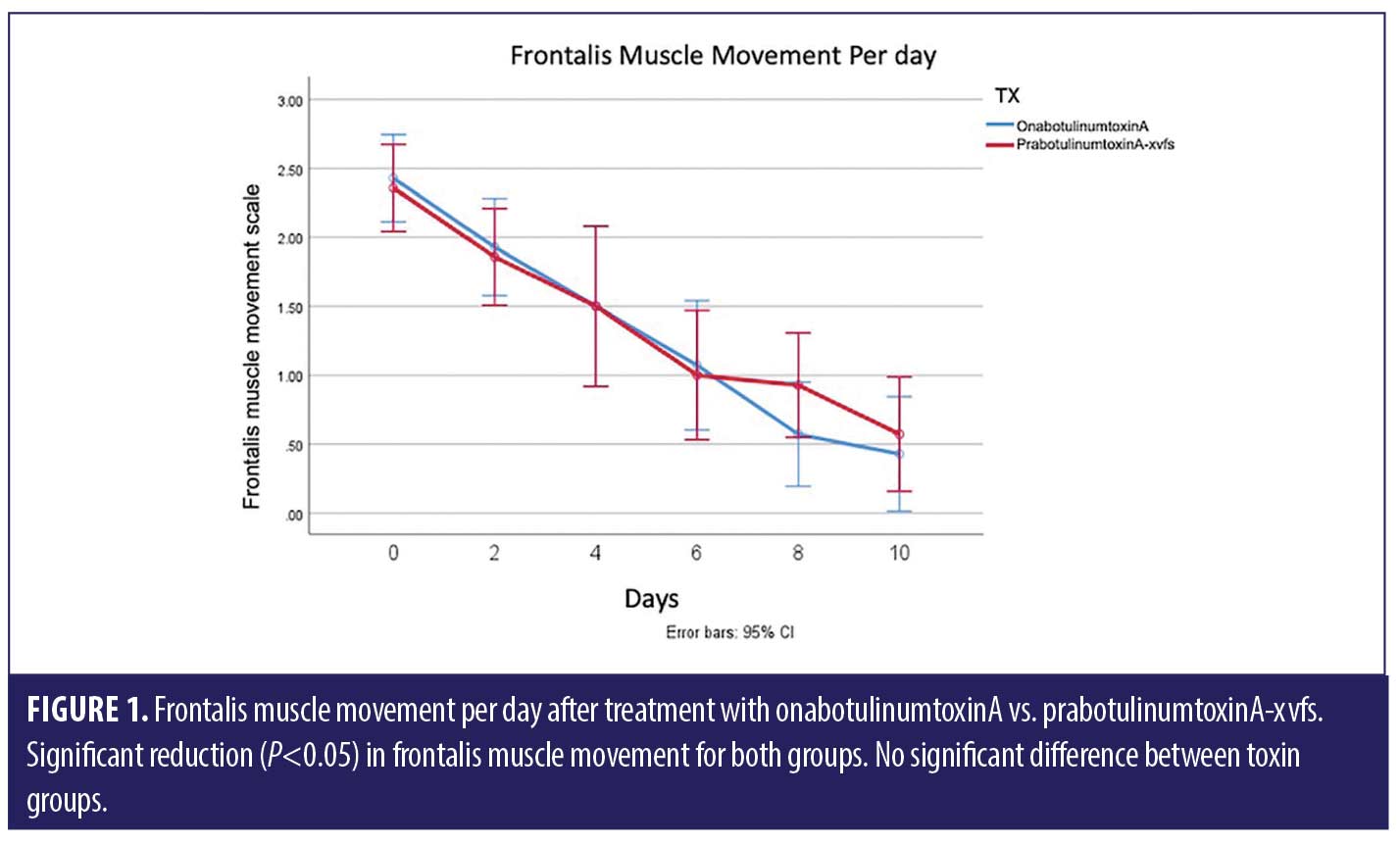
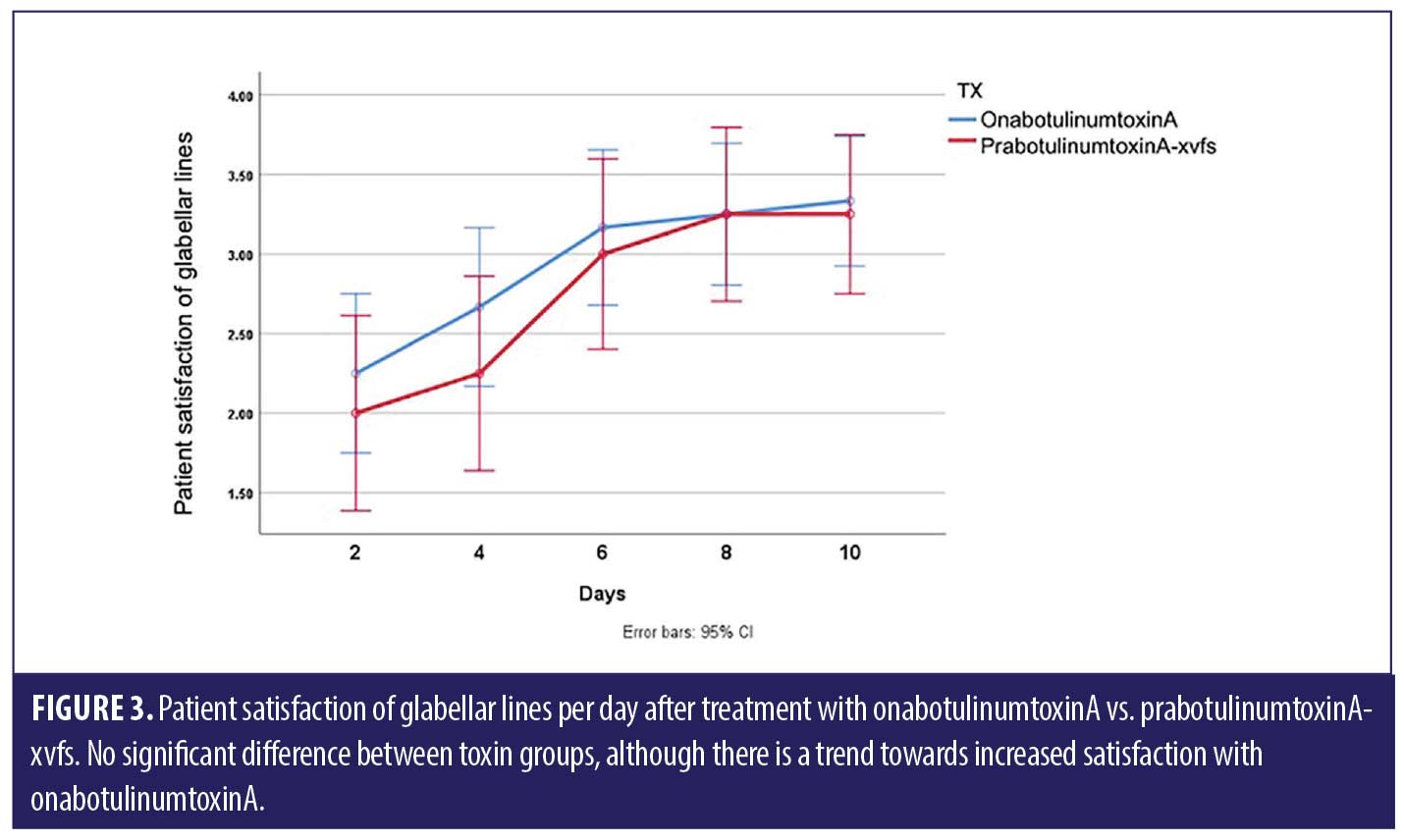
Results. There was no statistically significant difference in onset to action, rhytid appearance, and patient satisfaction after injection with onabotulinumtoxinA versus prabotulinumtoxinA-xvfs in the corrugator and frontalis muscles. Although not statistically significant, a trend existed towards increased patient satisfaction with onabotulinumtoxinA.
Conclusion. Both onabotulinumtoxinA and prabotulinumtoxinA-xvfs are equally efficacious formulations of botulinum toxin type A for the treatment of glabellar and forehead rhytids.
Keywords. Aging skin, antiaging, fillers, and injectables
The injection of neurotoxins for forehead and glabellar lines is an extremely common cosmetic procedure around the world. Both onabotulinumtoxinA and prabotulinumtoxinA-xvfs are United States Food and Drug Administration (FDA)-approved formulations of botulinum toxin A. OnabotulinumtoxinA has been available in the United States since 2002 as a temporary treatment for rhytids,1 while prabotulinumtoxinA-xvfs has more recently been made available in 2019.2 There have been no split-face comparison studies between the two formulations for rhytids of the glabella and forehead. This is the first triple-blind, randomized, split-face study comparing the onset of action, rhytid appearance, and patient satisfaction for forehead and glabellar lines of onabotulinumtoxinA and prabotulinumtoxinA-xvfs.
The aim of this study was to compare the onset to action and patient satisfaction of onabotulinumtoxinA and prabotulinumtoxinA-xvfs in treating dynamic rhytids of the forehead and glabella.
Methods
Baseline photographs of resting face, maximal frown lines, and maximal forehead raising lines were taken of each patient in similar lighting. OnabotulinumtoxinA (50-unit vial) was reconstituted with 1mL of sterile normal saline and prabotulinumtoxinA-xvfs (100-unit bottle) was reconstituted with 2mL of sterile normal saline by a blinded physician. Patients were randomly assigned by computer-generated randomization to receive both onabotulinumtoxinA and prabotulinumtoxinA-xvfs injected to opposite sides of the face in an alternating sequence from one patient to the next. Fifteen patients with dynamic rhytids were evaluated and received equal volumes of one toxin to each side of the face into the frontalis muscle and corrugator muscles. Injection volumes were unique to each patient and their underlying facial characteristics. Injections into the corrugator muscles and frontalis muscles were done intradermally by a single blind injector. After the initial injection on Day 0, patients used a camera phone to take daily digital photographs of their glabellar and forehead lines upon maximal exertion for 10 days.
All study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as revised in 2013, and was approved by our institution. Informed consent was obtained from all patients for being included in the study.
Two blinded evaluators performed subjective assessments using the Facial Wrinkle Scale (FWS). Each side of the patient’s glabella and forehead were evaluated using baseline and posttreatment photographs. Patients completed a daily satisfaction questionnaire on Days 0, 2, 4, 6, 8, 10 to self-assess their satisfaction with the treatment overall and subjective time to onset of the toxin on their right and left sides. Study endpoints were onset of action, reduction in rhytids, and patient satisfaction.
Inclusion criteria. Patients were included in the study if they met the following critera:
- Has a minimum six-month history of no botulinum toxin injections into the glabellar region and/or forehead
- Presence of dynamic rhytids in glabella or forehead
- No history of allergic reaction or negative adverse effects from botulinum toxin
- Exclusion criteria. Patients were excluded from the study if they met any of the following criteria:
- Previous facial cosmetic surgery, permanent fillers, or tissue augmentation with silicone
- Planning a facial cosmetic surgery procedure during study period or botulinum toxin injected into glabella or forehead within six months
- A history of allergic reaction or negative adverse effects from botulinum toxins.
- A history of myasthenia gravis, ALS, any neuromuscular disorder
- Pregnant or breastfeeding
Results
Fifteen patients, aged 28 to 74, were enrolled and completed the study. A total of 54.7 percent of the participants were female and 45.3 percent were male. The patient’s ethnic background were as follows: 37.2 percent Hispanic, 35 percent White, 18.2 percent Asian, and 9.6 percent Black.
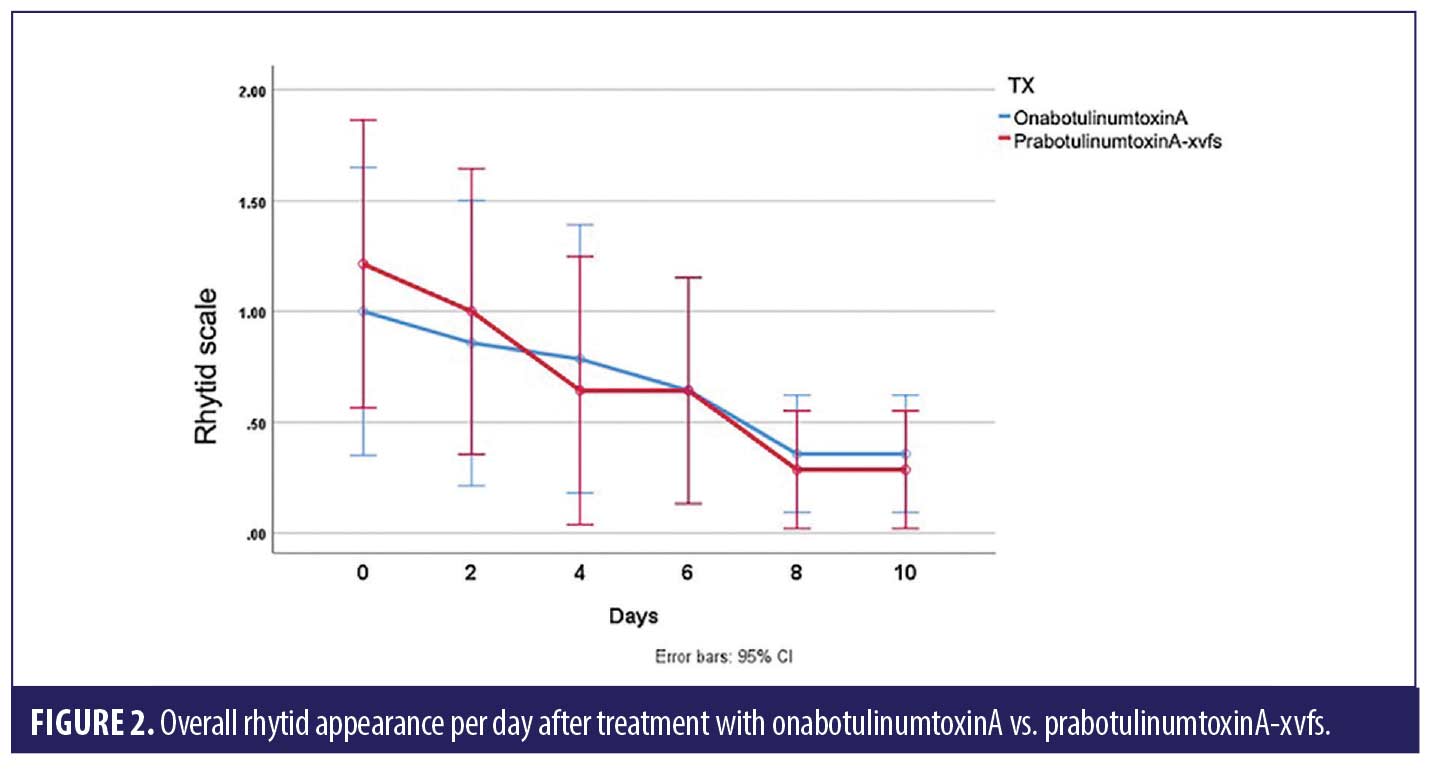
ANOVA with repeated measures analysis revealed that there was no statistically significant difference (p>0.05) in onset of action of onabotulinumtoxinA compared to prabotulinumtoxinA-xvfs when placed in the corrugator and frontalis muscles (Figure 1). There was no significant (p>0.05) difference in rhytid reduction (Figure 2) or satisfaction between study participants who received onabotulinumtoxinA versus prabotulinumtoxinA-xvfs, although a trend existed towards increased satisfaction of forehead rhytids with onabotulinumtoxinA (Figure 3).
Discussion
While onabotulinumtoxinA has been approved since 1980 for various medical conditions, it was not approved until 2002 for the cosmetic reduction of facial rhytids.1 Various alternatives to onabotulinumtoxinA have been developed; prabotulinumtoxinA-xvfs, the most recent alternative, was approved in 2019.2 Studies have shown that prabotulinumtoxinA-xvfs has similar longevity compared to other formulations of botulinum toxin A made available in the United States.3 One split-face study showed that prabotulinumtoxinA-xvfs and onabotulinumtoxinA have comparable efficacy and safety in the treatment of crow’s feet.3 Rzany et al4 reported that prabotulinumtoxinA-xvfs is effective and noninferior to onabotulinumtoxinA for the treatment of moderate to severe glabellar lines.4 There have been no split-face comparison studies of onabotulinumtoxinA versus prabotulinumtoxinA-xvfs in the forehead and glabella previously reported in the literature.
We found no statistically significant difference in onset of action for onabotulinumtoxinA compared to prabotulinumtoxinA-xvfs, which reveals non-superiority of one treatment over the other. As onset of action appears to be similar with both toxin types, we recommend considering the cost to the patient when choosing a toxin. In terms of patient satisfaction, there was no statistically significant difference between the two; however, there was a trend towards increased satisfaction with onabotulinumtoxinA.
Limitations of our study include a small sample size, as only 15 patients were enrolled and completed the study. No adverse effects were reported during the study, but four patients requested a touch up of toxin due to asymmetry after study completion. Of the four patients who requested a touch up, two of these patients requested touch ups in the side of the face where onabotulinumtoxinA was injected and two patients requested a touch up where prabotulinumtoxinA-xvfs was injected. No trend existed in terms of short-term follow-up asymmetry in our small-scale study based on these touch ups. Patients were followed for 10 days and long-term follow-up of patients was not completed.
Conclusion
Our study reveals that both onabotulinumtoxinA and prabotulinumtoxinA-xvfs are safe options for the reduction of facial rhytids, while having similar efficacy. A larger scale study is needed to compare the two different types of botulinum toxin A and to see if the data from our study is reproducible.
References
- Frampton JE, Easthope SE. Botulinum toxin A (Botox cosmetic): a review of its use in the treatment of glabellar frown lines. Am J Clin Dermatol. 2003;4:709–725.
- Beer KR, et al. Efficacy and safety of prabotulinumtoxinA for the treatment of glabellar lines in adult subjects: results from 2 identical phase III studies. Dermatol Surg. 2019; 45 (11): 1381–1393.
- Cheon HI, Jung N, Won CH et al. Efficacy and safety of prabotulinumtoxin A and onabotulinumtoxinA A for crow’s feet: A phase 3, multicenter, randomized, double-blind, split-face study. Dermatol Surg. 2019;45:1610.
- Rzany, BJ et al. A multicenter, randomized, double-blind, placebo-controlled, single-dose, phase III, non-inferiority study comparing prabotulinumtoxinA and onabotulinumtoxinA for the treatment of moderate to severe glabellar lines in adult patients. Aesthet Surg J. 2020; 40 (4): 413–429.

