 J Clin Aesthet Dermatol. 2021;14(7):38–41.
J Clin Aesthet Dermatol. 2021;14(7):38–41.
by Safoura Shakoei, MD; Manouchehr Nakhjavani, MD; Hossein Mirmiranpoor, MD; Mohana Alinejad Motlagh, MD; Arghavan Azizpour, MD; and Robabeh Abedini, MD
Drs. Shakoei and Motlagh are with the Department of Dermatology at Imam Khomeini Hospital, Tehran University of Medical Sciences in Tehran, Iran. Drs. Nakhjavani and Mirmiranpoor are with the Endocrinology and Metabolism Research Center at Vali-Asr Hospital, Tehran University of Medical Sciences in Tehran, Iran. Drs. Azizpour and Abedini are with the Department of Dermatology at Razi Hospital, Tehran University of Medical Sciences in Tehran, Iran.
FUNDING: This study was funded and supported by the Tehran University of Medical Sciences (grant no. 97-01-30-34656).
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Psoriasis is a chronic, immune-mediated, inflammatory disease. Previous studies have indicated a possible role of oxidative stress in the pathogenesis of psoriasis.
Objective. We sought to compare special oxidative stress and antioxidant markers in psoriatic patients.
Methods. This study included 35 patients with psoriasis and 35 healthy controls. Serum levels of oxidant markers, including advanced glycation end products (AGEs) and advanced oxidation protein products (AOPPs), as well as antioxidant enzymes, including lecithin-cholesterol acyltransferase (LCAT), paraoxonase-1 (PON1), and ferric-reducing ability of plasma (FRAP), were measured.
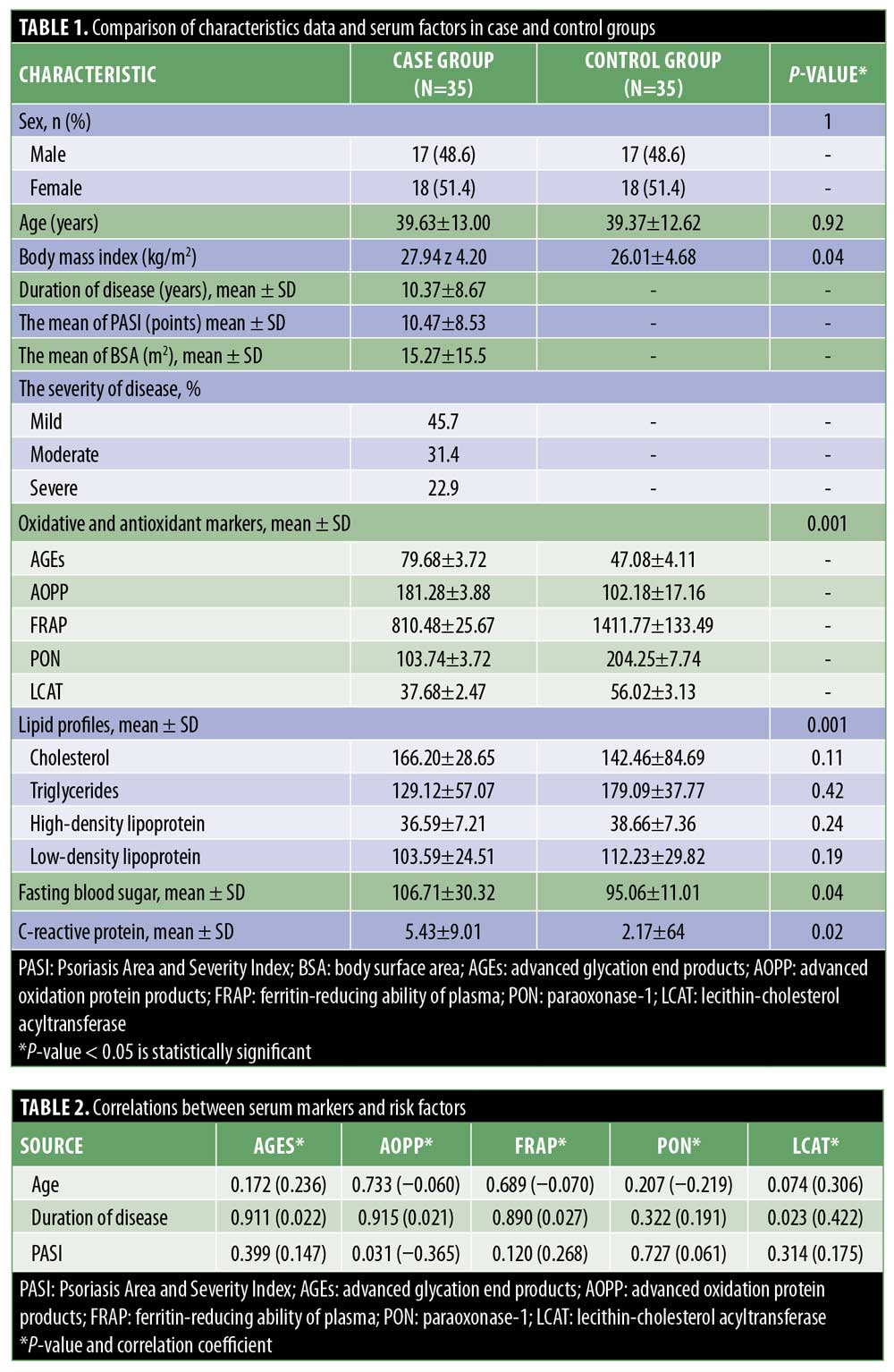
Results. The mean age of the subjects was 39.63±13 years in the case group and 39.37±12.62 years in the control group (p=0.92). The mean Psoriasis Area and Severity Index (PASI) scores of these groups were 15.27 and 10.47. The mean levels of fasting blood sugar and C-reactive protein were significantly higher in the case group than the control group (p=0.04 and p=0.02, respectively). Moreover, the mean levels of AGEs and AOPPs in the case group were significantly higher than in the control group (p=0.001), while the mean levels of FRAP, PON1, and LCAT were significantly lower in the case group than in the control group (p=0.001). There was no significant association between PASI and oxidant or antioxidant markers, except for AOPP, which had a negative association with PASI.
Conclusions. Our findings suggest an imbalance among oxidative stress and antioxidant markers in the pathogenesis of psoriasis. The oxidant-antioxidant enzymatic system is impaired in psoriasis as a result of increased oxidant products and reduced antioxidant activity.
Keywords: Psoriasis, advanced glycation end products, advanced oxidation protein products, lecithin-cholesterol acyltransferase, paraoxonase-1
Psoriasis is a chronic, immune-mediated, inflammatory disease that affects the skin and joints. The prevalence of this disease varies between 0.6 and 4.8 percent.1 Although it can present at any age from birth to older age, the peak age ranges of onset are 15 to 20 and 55 to 60 years.2 The exact etiology of psoriasis remains unclear. However, genetic, immunological, psychological, hormonal, and environmental factors might be involved. The pathogenesis of psoriasis might be associated with abnormal interactions between innate immune cells, T-cells, and keratinocytes; activation of the immune system; the release of excess pro-inflammatory substances; and tissue or organ damage.3,4
Psoriasis lesions are characterized by localized or widespread pruritic erythematous plaques with thick, silvery-white scales.5 Recently, several studies have evaluated the role of reactive oxygen species and oxidative stress in the pathogenesis of psoriasis. Oxidative stress by disruption of redox signaling might cause molecular damage.6,7 It can also activate dendritic cells, lymphocytes, and keratinocytes and increase the levels of enzymatic/nonenzymatic antioxidants and lipid peroxidation products, leading to angiogenesis, inflammation, cell necrosis, and apoptosis.8 Reactive oxygen species are oxidative stress markers. Pyrolyzed proteins, protein carbonyl compounds (PCCs), and advanced oxidation protein products (AOPPs) are some protein oxidative stress marker products. The characteristics of these markers can be quantified, including early production, stability, reliability, and long lifespan.8,9
Advanced glycation end products (AGEs) are produced through the oxidation of sugars, lipids, and amino acids and form aldehydes. Aldehydes can bind to proteins and accumulate in tissues, resulting in hyperglycemia, hyperlipidemia, and oxidative or carbonyl stress.10 On the other hand, lecithin-cholesterol acyltransferase (LCAT), paraoxonase-1 (PON1), and ferric-reducing ability of plasma (FRAP) as antioxidant factors can protect cells against lipid oxidation and the accumulation of oxidized low-density lipoprotein (LDL) and improve the antioxidant properties of high-density lipoprotein (HDL).11–13
Previous studies have indicated a possible role of oxidative stress and antioxidant markers in the pathogenesis of psoriasis, but there are still some controversies in this area. The objective of this cross-sectional study was to compare the serum levels of oxidative stress markers and antioxidant markers, including AGEs, LCAT, PON1, AOPPs, and FRAP, between patients with psoriasis and healthy controls. This study evaluated different oxidant and antioxidant factors among patients with psoriasis. Identification of some risk factors can provide more valuable information about the treatment of this disease.
Methods
This cross-sectional study was conducted in the dermatology outpatient departments of two hospitals between 2018 and 2019. Patients with a diagnosis of psoriasis were considered as the case group. The severity of disease was determined according to the Psoriasis Area and Severity Index (PASI).14 Total PASI scores of less than seven, 7 to 15, and greater than 15 represented mild, moderate, and severe psoriasis, respectively.15 Sex- and age-matched healthy individuals with cosmetic complaints were also recruited to the control group.
The exclusion criteria were as follows: 1) history of concurrent autoimmune or inflammatory diseases, immunodeficiency disorders, or cancers; 2) underlying diseases (e.g., diabetes, familial hypercholesterolemia, metabolic syndrome, renal disease, and liver dysfunction); 3) consumption of oral contraceptive pills, vitamin C or E, diuretics, or anti-inflammatory drugs in the past three months; 4) recent surgery; and 5) excessive exercises. Individuals with alcohol consumption or smoking habits, as well as patients undergoing systemic treatment in the past six months, were also excluded. The study population was informed about the study and asked to sign informed consent forms. Then, demographic and clinical data were gathered and recorded. The body mass index was calculated as a person’s weight in kilograms divided by the square of height in meters (kg/m2).
Laboratory measurements. Venous blood samples (10mL) were collected after overnight fasting. They were centrifuged for 15 minutes, stored frozen at -70°C, and sent to the laboratory. The serum levels of AOPPs, AGEs, and FRAP were determined via spectrofluorimetry. A commercially available kit (423901; Calbiochem, San Diego, California) was used to assess the LCAT activity by the fluorometric method. The serum PON1 level was also assessed using an automated paraoxonase assay kit (V31137; ZellBio, Berlin, Germany) by the colorimetric method.
Lipid profile and fasting blood sugar (FBS) were assayed by standard enzymatic colorimetric methods using enzymatic assays (Parsazmun Co. Ltd., Tehran, Iran). LDL was also calculated based on the Friedewald formula. The serum C-reactive protein (CRP) level was assessed in all subjects using a two-site enzyme-linked immunosorbent assay (Diagnostic Biochem, Ontario, Canada). Data were analyzed to compare the serum oxidative and antioxidant markers between the case and control groups as the primary outcome. Moreover, correlations between some demographic factors and concentrations of serum markers were evaluated in both groups.
Sample size. According to a study by Yazici et al,9 the mean levels of AOPPs were 62.4±20.6 and 36.1±13.2 umol/L among psoriasis patients and controls, respectively. The sample size was calculated to be 35 subjects per group. With a total sample size of 70 patients, the study had a power of 90 percent and an alpha error of 0.05.
Statistical analysis. All statistical analyses were conducted using the Statistical Package for the Social Sciences version 19 (IBM Corporation, Armonk, New York). Quantitative variables with a normal distribution were reported as mean ± standard deviation and percentage values, while quantitative variables without a normal distribution were presented as median, range (min–max), and quartile values. Qualitative variables were also reported as percentages. Also, the Mann-Whitney test, analysis of variance, Kruskal-Wallis test, Pearson’s correlation test, Spearman’s correlation test, and t-test were used to analyze the correlations between variables. P-values of less than 0.05 were considered to be statistically significant.
Ethical considerations. The present study was extracted from a medical student thesis. Ethical approval was obtained according to the international guidelines of the Declaration of Helsinki. Written informed consent was obtained from the included patients.
Results
This study was conducted on 35 patients with psoriasis and 35 subjects in the control group. In total, 18 (51.4%) women and 17 (48.6%) men were included in each group. The mean age of the subjects was 39.63±13 years in the case group (range: 17–77 years) and 39.37±12.62 years in the control group (range: 18–75 years) (p=0.92). The mean body mass index in the case group was significantly higher than that of the controls (27.94±4.20 vs. 26.01±4.68 kg/m2; p=0.04). Based on these findings, the mean age at onset of disease was 26.86±12.61 years (range: 9–57 years), and the mean disease duration was 10.37±8.67 years (range: 1–37 years).
The mean PASI score and body surface area (BSA) were 10.47 and 15.27m2, respectively. The mean levels of oxidative stress and antioxidant markers were significantly different between the two groups: the mean levels of AGEs and AOPPs were significantly higher in psoriasis patients (p=0.001), while the mean levels of FRAP, PON1, and LCAT were significantly lower in psoriasis patients (p=0.001). The mean FBS and CRP levels in the case group were also significantly higher than in the control group (p=0.04 and p=0.02, respectively). However, no significant differences were observed regarding the lipid profile between the groups (p>0.05). Detailed data of the case group are presented in Table 1.
The mean serum PON1 level was inversely correlated with the CRP level (r = -0.352; p=0.041). No significant relationship was found between CRP level and BSA (p=0.351) or PASI score (p=0.532). Also, the grade of psoriasis severity (mild, moderate, and severe) had no significant correlation with serum oxidative/antioxidant markers (p>0.05). Correlations between serum markers and some risk factors were also assessed in this study. Spearman’s correlation and Mann-Whitney tests showed that age and sex had no significant associations with oxidative stress or antioxidant markers in the case and control groups (p>0.05) (Table 2).
Discussion
The present findings demonstrated associations between psoriasis and serum levels of different oxidative stress and antioxidant biomarkers. According to the results, the mean level of AGEs in patients with psoriasis was significantly higher than that among healthy controls. It is known that psoriasis stimulates pro-inflammatory responses and chronic inflammation by activation of monocytes, macrophages, neutrophils, and endothelial cells. This activation of the immune system results in the production of more cytokines and reactive oxygen forms and the accumulation of AGEs.16
The role of AGEs has been confirmed in the pathogenesis of inflammatory diseases, such as diabetes and psoriasis.17
Oxidative stress is suggested to play a critical role in the pathogenesis of autoimmune disorders, including vitiligo, alopecia areata, and pemphigus vulgaris.7,18,19
Consistent with our findings, Damasiewicz-Bodzek et al16 reported a significant increase in AGE-peptide concentrations in patients with psoriasis compared to in healthy individuals. They concluded that there was a significant correlation between psoriasis and oxidative stress markers, such as protein glyco-oxidation products.16 Moreover, Papagrigorak et al20 showed that the serum levels of AGEs in patients with severe psoriasis were significantly higher than those of healthy controls.
The results of the present study demonstrated a significant increase in AOPPs in the case group relative to in the control group. Psoriasis may be responsible for endothelial dysfunction and vascular remodeling, resulting in cytokine imbalance and increasing the level of AOPPs. The association between psoriasis and increased serum levels of AOPPs has been evaluated in previous studies. In line with our findings, Haberka et al21 reported that AOPPs were significantly higher in patients with mild to moderate psoriasis compared to controls.
Yazici et al9 also indicated an increase in protein oxidation in mild to severe psoriatic patients. They demonstrated that psoriasis-related inflammation, phagocytic cell oxidation, and oxidation reactions of myeloperoxidase–hypochlorous acid could cause protein oxidation, reflected by increased levels of oxidative markers, such as AOPPs.9 Conversely, Skoie et al22 did not show any significant difference between patients with psoriasis and healthy subjects regarding plasma AOPP concentrations (p=0.75). Moreover, our results showed that AOPP concentration had negative associations with PASI and BSA. However, such correlations have not been reported by other research.9,22 The AOPP plasma level was increased in patients with alopecia areata compared to healthy controls; however, the difference was statistically insignificant.23
Based on the present results, the levels of FRAP, PON1, and LCAT as antioxidant biomarkers were significantly lower in the case group compared to in the control group. In line with our findings, Barygina et al24 showed a significant decrease in the mean total antioxidant capacity of psoriasis patients as compared with among controls. Some research has demonstrated a significant decrease in PON1 activity among patients with psoriasis and patients with alopecia areata compared to among healthy controls.25,26 Furthermore, Hashemi et al27 demonstrated that the plasma concentration of FRAP, as a total antioxidant capacity marker, was significantly lower in psoriatic patients than in healthy controls. Conversely, Esmaeili et al28 did not find any significant difference in terms of FRAP level between patients and controls. Holzer et al29 found that LCAT was reduced in patients with psoriasis and suggested a possible protective effect of this antioxidant biomarker against psoriasis. They showed that antipsoriatic therapy could increase PON1, which prevented LCAT inactivation.29
The results of the our study revealed that the mean FBS and CRP levels in patients with psoriasis were significantly higher than in healthy controls. Other studies have confirmed these findings as well. Farshchian et al30 demonstrated a significant increase in CRP level to be a systemic inflammatory biomarker in patients with psoriasis. Significantly higher levels of CRP were also reported by Vadakayil et al31 among patients with psoriasis compared to healthy controls. It seems that psoriasis, because of its inflammatory nature, might influence hormone production and insulin resistance, resulting in elevated blood sugar levels.32 In a study by Abedini et al,33 significantly higher levels of FBS were reported in 123 Iranian psoriatic patients compared to among healthy controls.33 However, this finding was not confirmed in the study by Farshchian et al, which also involved an Iranian population.34
Limitations. Our study was limited by its small sample size. Our observations must be confirmed in future studies with larger sample sizes.
Conclusion
Our findings revealed that an imbalance of oxidative stress and antioxidant factors might contribute to the pathogenesis of psoriasis. Higher levels of AGEs and AOPPs and lower levels of FRAP, PON1, and LCAT were significant among patients with psoriasis compared to the healthy controls. Therefore, treatment based on antioxidant strategies might be beneficial in psoriasis management.
References
- Egeberg A, Skov L, Gislason GH, Thyssen JP, Mallbris L. Incidence and prevalence of psoriasis in Denmark. Acta Derm Venereol. 2017;97(7):808–812.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64 Suppl 2:ii18–ii23; discussion ii4–ii5.
- Damasiewicz-Bodzek A, Kos-Kudla B. [Hormonal factors in aetiopathogenesis of psoriasis]. Pol Merkur Lekarski. 2007;22(127):75–78.
- Zhang P, Wu MX. A clinical review of phototherapy for psoriasis. Lasers Med Sci. 2018;33(1):173–180.
- Boehncke WH, Schon MP. Psoriasis. Lancet (London, England). 2015;386(9997):983–994.
- Lin X, Huang T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free Radic Res. 2016;50(6):585–595.
- Speeckaert R, Dugardin J, Lambert J, et al. Critical appraisal of the oxidative stress pathway in vitiligo: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2018;32(7):1089–1098.
- Chimenti MS, Sunzini F, Fiorucci L, et al. Potential role of cytochrome c and tryptase in psoriasis and psoriatic arthritis pathogenesis: focus on resistance to apoptosis and oxidative stress. Front Immunol. 2018;9:2363.
- Yazici C, Kose K, Utas S, et al. A novel approach in psoriasis: first usage of known protein oxidation markers to prove oxidative stress. Arch Dermatol Res. 2016;308(3):207–212.
- Papagrigoraki A, Maurelli M, Del Giglio M, et al. Advanced glycation end products in the pathogenesis of psoriasis. Int J Mol Sci. 2017;18(11).
- Litvinov D, Mahini H, Garelnabi M. Antioxidant and anti-inflammatory role of paraoxonase 1: implication in arteriosclerosis diseases. N Am J Med Sci. 2012;4(11):523–532.
- Glomset JA, Janssen ET, Kennedy R, Dobbins J. Role of plasma lecithin:cholesterol acyltransferase in the metabolism of high density lipoproteins. J Lipid Res. 1966;7(5):638–648.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76.
- Geale K, Henriksson M, Schmitt-Egenolf M. How is disease severity associated with quality of life in psoriasis patients? Evidence from a longitudinal population-based study in Sweden. Health Qual Life Outcomes. 2017;15(1):151.
- Llamas-Velasco M, de la Cueva P, Notario J, et al. Moderate psoriasis: a proposed definition. Actas Dermosifiliogr. 2017;108(10):911–917.
- Damasiewicz-Bodzek A, Wielkoszynski T. Advanced protein glycation in psoriasis. J Eur Acad Dermatol Venereol. 2012;26(2):172–179.
- Piwowar A. [Advanced oxidation protein products. Part I. Mechanism of the formation, characteristics and property]. Pol Merkur Lekarski. 2010;28(164):166–169.
- Bakry OA, Elshazly RM, Shoeib MA, Gooda A. Oxidative stress in alopecia areata: a case-control study. Am J Clin Dermatol. 2014;15(1):57–64.
- Yesilova Y, Ucmak D, Selek S, et al. Oxidative stress index may play a key role in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2013;27(4):465–467.
- Papagrigoraki A, Del Giglio M, Cosma C, et al. Advanced glycation end products are increased in the skin and blood of patients with severe psoriasis. Acta Derm Venereol. 2017;97(7):782–787.
- Haberka M, Banska-Kisiel K, Bergler-Czop B, et al. Mild to moderate psoriasis is associated with oxidative stress, subclinical atherosclerosis, and endothelial dysfunction. Pol Arch Intern Med. 2018;128(7-8):434–439.
- Skoie IM, Dalen I, Omdal R, Jonsson G. Malondialdehyde and advanced oxidation protein products are not increased in psoriasis: a controlled study. Arch Dermatol Res. 2019;311(4):299–308.
- Cwynar A, Olszewska-Slonina D, Czajkowski R, et al. Evaluation of selected parameters of oxidative stress in patients with alopecia areata. Postepy Dermatol Alergol. 2019;36(1):115–116.
- Barygina VV, Becatti M, Soldi G, et al. Altered redox status in the blood of psoriatic patients: involvement of NADPH oxidase and role of anti-TNF-alpha therapy. Redox Rep. 2013;18(3):100–106.
- Bacchetti T, Simonetti O, Ricotti F, et al. Plasma oxidation status and antioxidant capacity in psoriatic children. Arch Dermatol Res. 2020;312(1):33–39.
- Dizen-Namdar N, Emel Kocak F, Kidir M, et al. Evaluation of serum paraoxonase, arylesterase, prolidase activities and oxidative stress in patients with alopecia areata. Skin Pharmacol Physiol. 2019;32(2):59–64.
- Hashemi M, Mehrabifar H, Daliri M, Ghavami S. Adenosine deaminase activity, trypsin inhibitory capacity and total antioxidant capacity in psoriasis. J Eur Acad Dermatol Venereol. 2010;24(3):329–334.
- Esmaeili B, Mansouri P, Doustimotlagh AH, Izad M. Redox imbalance and IL-17 responses in memory CD4(+) T cells from patients with psoriasis. Scand J Immunol. 2019;89(1):e12730.
- Holzer M, Wolf P, Inzinger M, et al. Anti-psoriatic therapy recovers high-density lipoprotein composition and function. J Invest Dermatol. 2014;134(3):635–642.
- Farshchian M, Ansar A, Sobhan M, Hoseinpoor V. C-reactive protein serum level in patients with psoriasis before and after treatment with narrow-band ultraviolet B. An Bras Dermatol. 2016;91(5):580–583.
- Vadakayil AR, Dandekeri S, Kambil SM, Ali NM. Role of C-reactive protein as a marker of disease severity and cardiovascular risk in patients with psoriasis. Indian Dermatol Online J. 2015;6(5):322–325.
- Sondermann W, Djeudeu Deudjui DA, Korber A, et al. Psoriasis, cardiovascular risk factors and metabolic disorders: sex-specific findings of a population-based study. J Eur Acad Dermatol Venereol. 2020;34(4):779–786.
- Abedini R, Salehi M, Lajevardi V, Beygi S. Patients with psoriasis are at a higher risk of developing nonalcoholic fatty liver disease. Clin Exp Dermatol. 2015;40(7):722–727.
- Farshchian M, Zamanian A, Farshchian M, et al. Serum lipid level in Iranian patients with psoriasis. J Eur Acad Dermatol Venereol. 2007;21(6):802–805.

