 J Clin Aesthet Dermatol. 2022;15(8):47–51.
J Clin Aesthet Dermatol. 2022;15(8):47–51.
by Angie Ariza-Hutchinson, MD; Rosemina A. Patel, MD; N. Suzanne Emil, MD; Maheswari Muruganandam, MD; Sharon E. Nunez, MD; Matthew K. McElwee, MD; Frank X. O’Sullivan, MD; Roderick A. Fields, MD;
William A. Hayward, MD, Phd; Luke J. Haseler, MD; and Wilmer L. Sibbitt JR., MD
Drs. Hutchinson, Patel, Emil, Muruganandam, Nunez, McElwee, O’Sullivan, Fields, and Sibbitt are with the Department of Internal Medicine, Division of Rheumatology and School of Medicine at the University of New Mexico Health Sciences Center in Albuquerque, New Mexico. Dr. Hayward is with the Department of Exercise and Sport Sciences at New Mexico Highlands University in Las Vegas, New Mexico. Dr. Haseler is with the Faculty of Health Sciences, School of Physiotherapy and Exercise Science at Curtin University in Perth, Australia.
ABSTRACT: Objective. Rheumatoid nodules (RN), a classic cutaneous extra-articular manifestation of rheumatoid arthritis, can often cause discomfort or cosmetic embarrassment. This research determined the effectiveness and complications of corticosteroid injection of the RN.
Methods. Using a repeated measure design, 66 consecutive symptomatic RN were measured, underwent corticosteroid injection with 1 to 2mL of a 50:50 mixture of 1% lidocaine and triamcinolone acetonide (20–40mg), and then reassessed at four months for softening, reduction in size, and complications, including infection.
Results. The mean age of our patient group was 53.3±10.6 years; 45 percent were Hispanic, 55 percent were non-Hispanic White, 100 percent were seropositive (rheumatoid factor and/or anti-CCP antibody), and 87.5 percent were female. Baseline mean RN diameter was 0.50±0.51cm and four months after injection was reduced to 0.29±0.33cm (decreased 42% or 0.21±0.57cm reduction, 95% CI: 0.46 <0.21< 0.37, p=0.013), 100 percent (66/66) were less painful, and 77 percent (51/66) were palpably softened. However, 70 percent (46/66) demonstrated cutaneous atrophy and/or hypopigmentation at four months, 53 percent (35/66) nodules recurred within 12 months, and 47 percent (31/66) nodules were eventually surgically removed.
Limitations. Two (3%) of the larger RN (2.5cm on the olecranon and 2cm on the 2nd toe) became infected and failed antibiotic therapy, necessitating surgical excision for complete resolution.
Conclusion. For short-term symptomatic relief, smaller RN can be safely injected with triamcinolone. Large symptomatic RN (≥2cm) are at greater risk of infection; thus, in these cases, lower corticosteroid doses or surgical excision may be preferred. In the long-term, effective systemic antirheumatic therapy with treat-to-target is the best approach.
Keywords: Rheumatoid nodule, injection, rheumatoid arthritis, infection, corticosteroid
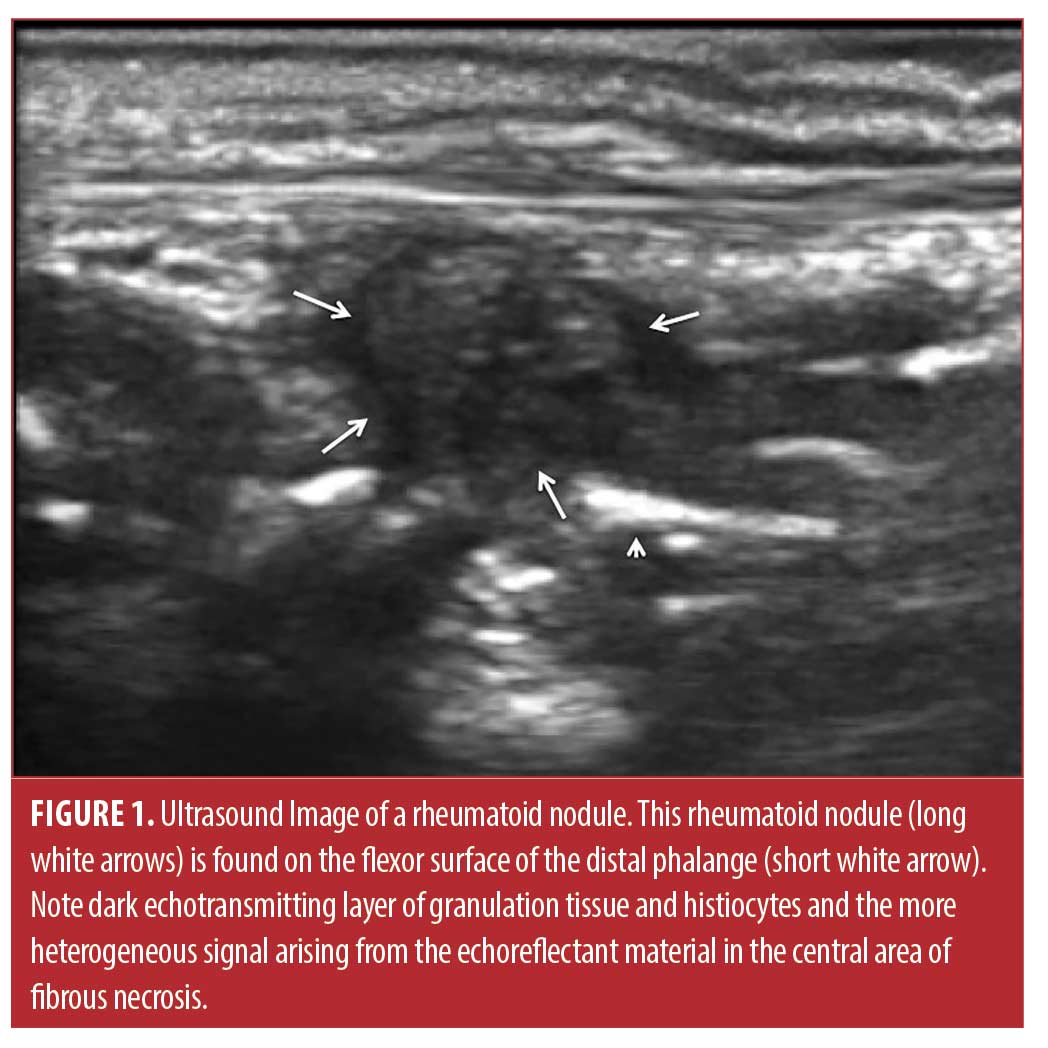
Rheumatoid nodules, the classic cutaneous manifestation of rheumatoid arthritis (RA), are associated with seropositivity, vasculitis, erosion, extraarticular complications, and more severe disease, but must be differentiated from gout tophi, sebaceous cysts, foreign body granulomas, multicentric reticulohistocytosis, and other confounding skin lesions.1-3 The nodules are typically firm, nontender, and freely movable within the subcutaneous tissue, although they can be attached to deeper structures such as periosteum, fascia, or tendons.2 They are usually from 2mm to greater than 5cm in diameter (Figure 1). Rheumatoid nodules tend to arise in areas subject to pressure or trauma, particularly over bony prominences. Thus, these lesions are found most commonly on extensor surfaces such as the olecranon process and the proximal portion of the ulna. Other locations frequently involved include the metacarpophalangeal and proximal interphalangeal joints, the ischial tuberosities, various joints in the foot, and the sacrum. Although rheumatoid nodules are usually asymptomatic, they can cause pain, can be easily traumatized, causing non-healing ulcers, and commonly result in cosmetic embarrassment for the patient.
To resolve rheumatoid nodulosis, effective antirheumatic systemic therapy for RA and cessation of cigarette smoking are always indicated, and resolution or reduction in size of nodules is often observed with effective therapy.4-7 However, certain systemic therapies for RA may actually provoke rheumatoid nodules, especially methotrexate and anti-tumor necrosis factor drugs.8-10 Thus, if the patient is otherwise responding to anti-rheumatic therapy, surgery is often used for symptomatic or troublesome rheumatoid nodules, especially if ulcerated or infected.2,3,11,12 Another alternative is injection of the rheumatoid nodule with fluorouracil or corticosteroid, treatments that have been shown to decrease nodule size.13,14 Corticosteroid alone is most commonly injected into the nodules, and has been shown to be effective by pioneering studies of Ching et al and Baan et al, but there has been concern about subsequent infection.2,14-17 The incidence of infection after corticosteroid injection is unclear as the study by Ching et al injected 24 nodules and the study by Baan et al nine nodules with corticosteroid with no reported infections, implying an infection rate of three percent or lower.2,14-17 In the present study, we injected a larger number of rheumatoid nodules of various sizes specifically to determine clinical response rates, long-term outcome, and complications, including infection.
Methods
Inclusion and exclusion criteria and study structure. This project was approved by the institutional review board (IRB) (Human Protections Office of the University of New Mexico Health Sciences Center, Albuquerque, New Mexico, USA). All studies were carried out in accordance with the World Medical Association Declaration of Helsinki (JBJS 79A:1089-98,1997). Patient confidentiality was protected according to the U.S. Health Insurance Portability and Accountability Act (HIPAA) and all data has been de-identified. Using a clinical care repeated measure design where each nodule served as its own control, we injected consecutively 66 rheumatoid nodules in eight RA patients over a 10-year period and the data was logged into our procedure database.The IRB permitted retrospective analysis of our procedure database and medical records to determine outcomes. Inclusion criteria for nodule injection were 1) individual 18 years of age or older, 2) the diagnosis of RA, 3) the presence of symptomatic or cosmetically displeasing rheumatoid nodules, and 4) the desire of the patient to have the nodule injected rather than surgically removed. Exclusion criteria were 1) impaired, incarcerated, or pregnant individuals, 2) individuals younger than 18 years, 3) the presence of an erosion or infection on or in the rheumatoid nodule or overlying skin, or 4) allergy or intolerance to lidocaine or triamcinolone acetonide. RA was classified using the American College of Rheumatology/European League of Rheumatism 2010 Rheumatoid Arthritis Classification Criteria.18
All patients complained of a rheumatoid nodule that was painful, awkward, or cosmetically offensive and wished to have the nodule injected rather than removed surgically (Figures 1 and 2). All participants signed informed consent before any examination or procedures.
Patients were examined on the day of the procedure and four months later. Nodule diameter was determined by palpation in the longest axis and was expressed in centimeters (cm). Long-term follow up and the interventions of excisional surgery or reinjection were determined by patient interview and retrospective medical chart review. Complications including adverse medication reactions, skin depigmentation or atrophy, hematoma, or infection were recorded.

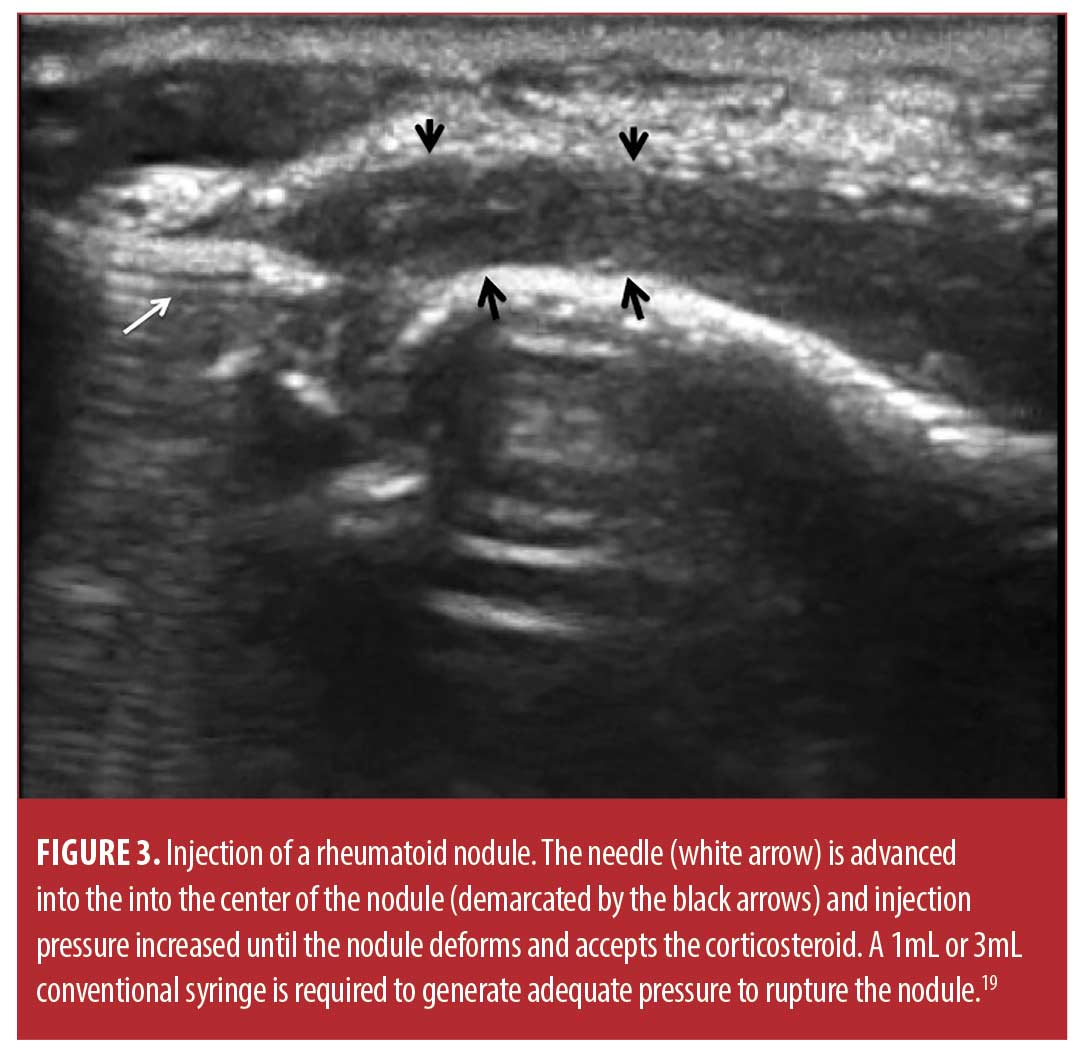
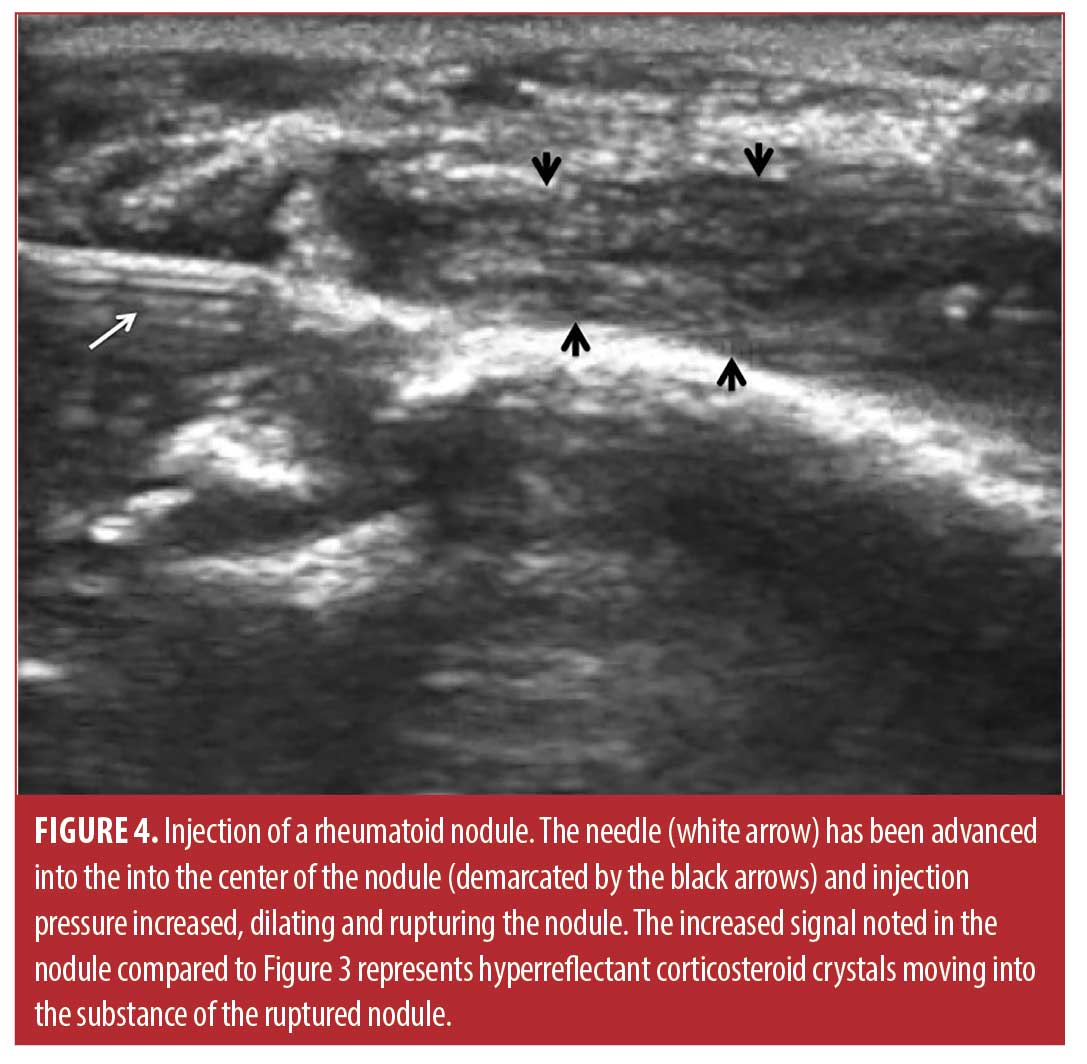
Injection technique. The sixty-six rheumatoid nodules consisted of 18 nodules on the thumb, 31 nodules on fingers other than the thumb, 13 nodules over the olecranon process of the elbow, and four nodules on the foot or toes. The rheumatoid nodule was palpated and marked with ink (Figure 2). The skin was first cleaned with chlorhexidine 2% for antisepsis. Depending on the diameter and depth of the nodule, a 25 gauge 5/8 inch (15.9mm) or 1.5 inch (38.1mm) needle (BD, Franklin Lakes, New Jersey) was mounted on a 1- or 3-mL syringe (1- or 3mL Luer Lok syringe, BD) filled with 1ml of 1% lidocaine (Xylocaine® 1%, AstraZeneca Pharmaceuticals LP, Wilmington, Delaware) and 1mL (40mg) triamcinolone acetonide (Kenalog® 40, Westwood-Squibb Pharmaceuticals, Inc, New York, New York). Since there is no real or potential space in a rheumatoid nodule, a 1- or 3-mL syringe was necessary to generate adequate pressure to inject into and rupture the resistant substance of the rheumatoid nodule.19 The 25g needle was introduced through the skin and through the proximal tissue into the substance of the rheumatoid nodule (Figures 2 and 3). Aspiration was then attempted and if there was no fluid or blood yield, the lidocaine/triamcinolone was then injected into the nodule until the nodule fractured (ruptured and dilated with injectate) (Figure 4). 1 mL (20mg triamcinolone acetonide in 0.5mL of 1% lidocaine) was used on nodules 2cm or less in diameter, and 2mL (40mg triamcinolone acetonide in 0.5ml of 1% lidocaine) was used on nodules greater than 2cm in diameter. The needle was then extracted, and firm pressure applied to the puncture site. The puncture area was washed with chlorhexidine and a sterile adhesive bandage was applied and then reassessed at four months for softening, reduction in size, and complications, including infection.
Data analysis. Data were entered into Excel (Version 5, Microsoft, Seattle, Washington), and analyzed in Simple Interactive Statistical Analysis (SISA) (Consultancy for Research and Statistics, Hilversum, The Netherlands). Descriptive statistics were used to summarize the demographic and clinical characteristics. Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were expressed as total number with percentiles. Measurement data was analyzed using the Student t-Test calculating both p values and confidence intervals.
Results
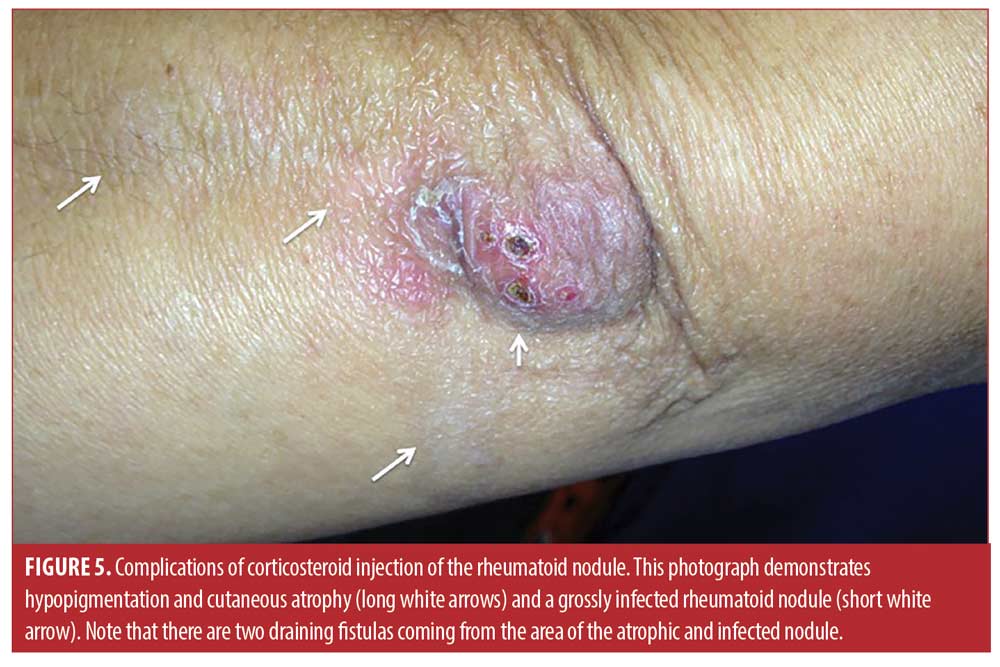
The mean age of the rheumatoid nodule cohort was 53.3±10.6 years, 45 percent were Hispanic, 55 percent were non-Hispanic White, 100 percent were seropositive (rheumatoid factor and/or anti-CCP antibody), and 87.5 percent were female. Baseline mean rheumatoid nodule diameter was 0.50±0.51cm and decreased to 0.29±0.33cm four months after the injection (a reduction of 42% or 0.21±0.57 cm; 95% CI: 0.46 <0.21< 0.37, p=0.013). One hundred percent (66/66) were less painful, and 77 percent (51/66) were palpably softened. However, 70 percent (46/66) demonstrated cutaneous atrophy or skin depigmentation at four months (Figure 5). Moreover, 53 percent (35/66) nodules recurred within 12 months, 50 percent (33/66) were re-injected, and 47 percent (31/66) nodules were eventually surgically excised. Two (3%) of the larger rheumatoid nodules (2.5cm on the olecranon and 2cm on the second toe) became infected with methicillin-sensitive Staphylococcus aureus and were treated unsuccessfully with antibiotics (Figure 5). Subsequent surgical excision was required with subsequent complete resolution.
Discussion
Hollingworth first described the technique of corticosteroid injection to decrease the size of rheumatoid nodules in 1968.17 Later Ching et al15 using injected methylprednisolone and Baan et al16 using injected triamcinolone described statistically significant decreases in the size of rheumatoid nodules in controlled studies. The present study using a paired repetitive measure design confirms that corticosteroid injection of rheumatoid nodules decreases the size (diameter) of rheumatoid nodules. The present study described a 42-percent decrease in nodule size, 100 percent reduction in pain, and 77 percent palpable softening of the rheumatoid nodule. This is similar to the 25 to 50 percent reduction in nodule size and pain reported by Ching et al,15 and the 30 to 80 percent reduction in size and pain reported by Baan et al.16 Neither previous study reported actual softening of the rheumatoid nodule in addition to reduced size, although this is somewhat a subjective determination. Injection of rheumatoid nodules with fluorouracil has also been reported, but this technique has never become popular for treating rheumatoid nodules, due to the perceived toxic nature of fluorouracil.13,14
Injection of rheumatoid nodules is not routinely performed in contemporary medicine for a number of reasons. First, modern systemic therapy of rheumatoid arthritis with disease modifying antirheumatic drugs (DMARDS) and biologics have been shown to reduce the size and number of rheumatoid nodules, presumably from decreasing systemic disease activity, thus suppressing the processes that drive rheumatoid nodule formation.4-9 For example, corticosteroids, sulfasalazine, colchicine, penicillamine, tocilizumab, and rituximab amongst others have been reported to decrease nodule size and numbers.2-8 On the other hand, methotrexate, infliximab, and cigarette smoking have been reported to increase rheumatoid nodule formation in some patients.7-10 Moreover, in contemporary RA treat-to-target strategies recommend either placing patients on combination antirheumatic therapy or switching to a completely different drug regimen in patients who develop rheumatoid nodules or other extraarticular manifestations.20 Thus, many or most patients will eventually be placed on antirheumatic therapy that has the potential to reduce rheumatoid nodule formation, and thus direct injection treatment of the nodule is often not necessary.2-8, 20
Another reason that injection of rheumatoid nodules is not frequently performed is the concern of infection resulting from the injection.2,7,15-17 Infections are theoretically more likely in the rheumatoid nodule due to the structure of the nodule, which is that of a granuloma.1-3 As recently reported in the excellent histopathologic study of Shin and colleagues,3 a typical rheumatoid nodule consists of an external layer of highly vascular granulation tissue with perivascular lymphocytic infiltration, a layer of less vascularized stromal fibrosis and palisading histiocytes, and an avascular center or cleft of fibrinoid necrosis containing immunoglobulins and other proteins, often complicated by cystic degeneration. If the avascular fibrinoid necrosis or cystic area becomes infected, it may be resistant to antibiotic because of the absence of a resident blood supply, and chronic draining fistulae may result (Figure 5).2, 3,12
The prior studies of Ching et al15 and Baan et al16 reported a total of 33 rheumatoid nodules injected with corticosteroid with no infections. While encouraging, these small numbers of cases would not typically detect an infection rate below three percent and did not reflect other previous publications in which infections and fistulae after corticosteroid injection of rheumatoid nodules were documented.1,2,15-17 In this study, 66 nodules were injected and two infections (3%) were noted, both infections in nodules 2cm in diameter or larger (one an olecranon and one a toe nodule). Both rheumatoid nodules were infected with methicillin-sensitive Staphylococcus aureus and resulted in fistulae formation, were clinically resistant to antibiotic therapy, and required complete excision of the infected nodule for complete resolution. Infections associated with corticosteroid injections can be widely diverse and can include common organisms such as Staphylococcus or Streptococcus, or may be polymicrobial, or involve unusual fungal or bacterial species.21,22
Other reasons that corticosteroid injection of rheumatoid nodules is not routinely performed are the complications of skin depigmentation and dermal atrophy (Figure 5).23 In the present study, 70 percent (46/66) demonstrated cutaneous atrophy or skin depigmentation at three months which is considerable, although this complication is generally reversible. It is likely that the larger triamcinolone dose (20-40mg) used in the present study contributed to this complication, as Ching et al used lower doses (4-10mg) and did not note this level of atrophy or depigmentation.15 Moreover, in the present study, injected rheumatoid nodules tended to recur.2 The present study demonstrated that of the 66 nodules injected 53 percent (35/66) nodules recurred within 12 months likely due to incomplete systemic disease control. Thus, in the present study 47 percent (31/66) nodules were eventually surgically removed after multiple injections, again emphasizing the tendency for the nodules to recur in the setting of higher systemic disease activity.
Limitations. This was not a randomized controlled study, but a case series. Nonetheless, the evidence regarding complications of corticosteroid injection of rheumatoid nodules is likely reliable. Also, the injected nodules occurred in various areas of the body, rather than in a standardized anatomic location. Alternatively, these variable anatomic locations closely resemble the typical nodule locations of a patient with RA and nodulosis seen in the outpatient setting. As is common practice, multiple symptomatic nodules were injected in the same patient, potentially confounding the effects of local corticosteroid versus systemic corticosteroid effects on individual nodules. Finally, the current treat-to-target strategies may suppress rheumatoid nodules through more effective systemic therapy of the underlying rheumatoid process, reducing the need for directed rheumatoid nodule treatment.20
Conclusion
For short-term symptomatic relief, rheumatoid nodules can generally be safely injected with corticosteroid. In the long-term effective treat-to-target systemic antirheumatic therapy is the preferable choice. Large nodules (≥2cm) are at greater risk of infection; in these larger symptomatic nodules the use of lower corticosteroid doses, watchful waiting for effective systemic RA suppression with treat-to-target strategies, or initial surgical excision may be preferred.
Acknowledgments
The authors thank Ms. Jackie Cremar for technical assistance in preparation of this article.
References
- Codriansky KA, Rünger TM, Bhawan J,et al. Multicentric reticulohistiocytosis: a systemic osteoclastic disease? Arthritis Rheum. 2008;15:59:444–448.
- Sibbitt WL Jr, Williams RC Jr. Cutaneous manifestations of rheumatoid arthritis. Int J Dermatol. 1982;21:563–572.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041–3048.
- Braun MG, Wagener P. Regression von peripheren und pulmonalen Rheumaknoten unter Rituximab-Therapie [Regression of peripheral and pulmonary rheumatoid nodules under therapy with rituximab]. Z Rheumatol. 2013;72:166–171.
- Englert HJ, Hughes GR, Walport MJ. Sulphasalazine and regression of rheumatoid nodules. Ann Rheum Dis.1987;46:244–225.
- Al Attia HM, Abushawish M. Treatment with tocilizumab leads to the disappearance of olecranon rheumatoid nodules. Int J Dermatol. 2012;51:197–198.
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601–606.
- Abraham Z, Rozenbaum M, Rosner I. Colchicine therapy for low-dose-methotrexate-induced accelerated nodulosis in a rheumatoid arthritis patient. J Dermatol. 1999;26:691–694.
- Houlder EL, Millier MJ, Highton J, et al. Expression of the genes facilitating methotrexate action within subcutaneous rheumatoid nodules. Clin Exp Rheumatol. 2017;35:943–947.
- Mackley CL, Ostrov BE, Ioffreda MD. Accelerated cutaneous nodulosis during infliximab therapy in a patient with rheumatoid arthritis. J Clin Rheumatol. 2004;10:336–338.
- Munns JJ, Ruff ME. Rheumatoid nodules. J Hand Surg Am. 2014;39:765–767.
- Swezey RL. The management of rheumatoid nodules. Am J Orthop. (Belle Mead NJ) 1997;26:73.
- Amini S, Baum B, Weiss E. A novel treatment for rheumatoid nodules (RN) with intralesional fluorouracil. Int J Dermatol. 2009;48:543–546.
- Diniz Mdos S, Almeida LM, Machado-Pinto J, et al. Rheumatoid nodules: evaluation of the therapeutic response to intralesional fluorouracil and triamcinolone. An Bras Dermatol. 2011;86:1236–1238.
- Ching DW, Petrie JP, Klemp P, et al. Injection therapy of superficial rheumatoid nodules. Br J Rheumatol. 1992;31:775–777.
- Baan H, Haagsma CJ, van de Laar MA. Corticosteroid injections reduce size of rheumatoid nodules. Clin Rheumatol. 2006;25:21–23.
- Hollingsworth JW. Subcutaneous nodules (granulomas). In: Local and systemic complications of rheumatoid arthritis. Philadelphia: WB Saunders, 1968:25.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581.
- Hayward WA, Haseler LJ, Kettwich LG, et al. Pressure generated by syringes: implications for hydrodissection and injection of dense connective tissue lesions. Scand J Rheumatol. 2011;40:379–382.
- Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73:1108–1123.
- Skedros JG, Henrie MK, Finlinson ED, et al. Polymicrobial anaerobic infection with a deep abscess in the supraspinous fossa following a subacromial corticosteroid injection. BMJ. Case Rep 2018;11:e226598.
- Teoh KH, Jones SA, Gurunaidu S, et al. Methicillin-resistant Staphylococcus aureus infection of the subacromial bursa: an unusual complication following subacromial corticosteroid injection (a report of two cases). Shoulder Elbow. 2015;7:182–186.
- Hayward WA, Sibbitt WL, Sibbitt RR, et al. Intralesional injection of triamcinolone acetonide for subcutaneous lipoma causing musculoskeletal and neurologic Symptoms. J Clin Aesthet Dermatol. 2018;11:38–42.

