 J Clin Aesthet Dermatol. 2021;14(9):12–16.
J Clin Aesthet Dermatol. 2021;14(9):12–16.
by Orit Markowitz, MD, and Cynthia X. Chan, BS
Dr. Markowitz is with the Department of Dermatology, Mount Sinai in New York, New York. Ms. Chan is with the Geisel School of Medicine at Dartmouth College in Hanover, New Hampshire.
FUNDING: A research grant was provided by Bausch Health.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Onychomycosis affects 43 million people in the United States and Europe. Topical therapies can be effective but require lengthy and costly regimens without an established method for predicting the treatment outcome.
Objective. We studied the role of optical coherence tomography (OCT), a noninvasive imaging device approved by the United States Food and Drug Administration, in evaluating onychomycosis treatment outcomes.
Methods. This investigator-initiated prospective clinical study recruited subjects from two urban medical centers. A total of 34 subjects with mild-to-moderate onychomycosis, confirmed using periodic acid-Schiff (PAS) staining, were treated with topical efinaconazole for 48 weeks, at which time a final PAS result was obtained.
Results. A positive final PAS result was associated with fungal findings on OCT and with nonclearance of fungal findings by week 12. A negative final PAS was associated with the absence of fungal findings by week 12. OCT findings at Week 8 may suggest a 77% chance of mycological nonclearance or 82% chance of clearance. These predictive values are higher than the currently documented mycological cure rate of 54%. Clinical examination data had no predictive value alone or in concert with OCT.
Conclusion. We recommend OCT imaging be performed at the end of the second month of treatment to inform shared decision-making regarding whether or not to continue efinaconazole for nine additional months. OCT’s ability to evaluate onychomycosis outcome in patients on topical efinaconazole both earlier and more reliably than nonimaging variables may improve the care given to and reduce the cost of onychomycosis for millions of patients.
Keywords: Optical coherence tomography, toenail, onychomycosis, efinaconazole, fungus
Onychomycosis is the most common nail disorder, affecting 4% of Americans and Europeans (approximately 43 million people). Most cases involve toenails as opposed to fingernails and are of the distal lateral subungual onychomycosis (DLSO) subtype (86%).1,2 Left untreated, onychomycosis may extend locally, leading to destruction, deformity, and decreased quality of life.3 The logistical costs of treatment can be high, with each 8-mL bottle of topical efinaconazole costing approximately US$1,200.4
Topical therapies are effective for mild-to-moderate onychomycosis but are limited by lengthy and costly regimens that negatively impact initiation and adherence. For example, efinaconazole topical solution 10% has been shown to be effective in 54% of cases,5 but there is no established method for predicting outcome before the nearly year-long treatment regimen ends. Mid-treatment potassium hydroxide wet-mount may highlight residual nonviable fungal fragments, and mid-treatment fungal stains have a sensitivity of at most 60%.6
Optical coherence tomography (OCT) is a noninvasive light-based imaging device approved by the United States Food and Drug Administration that can take cross-sectional and en-face images of cutaneous structures in-vivo and in real time.7 It has already demonstrated a diagnostic capability for onychomycosis,8,9 but its role in treatment has yet to be examined. This study assesses OCT’s ability to evaluate the effect of topical efinaconazole on onychomycosis, most importantly to help evaluate patient outcomes within two months of treatment.
Methods
This multicenter, prospective, investigator-initiated clinical trial recruited subjects from two urban sites in New York City: the Veterans Affairs (VA) New York Harbor Healthcare System and Mount Sinai Health System. Enrollment occurred from June 2016 to August 2018.
Subjects who presented to the dermatology or podiatry clinic at the two study sites with at least one great toenail (GTN) clinically suspicious for mild-to-moderate DLSO, defined as 20% to 50% clinical involvement of the target GTN, were screened. The target GTN was either the GTN with greater involvement or the right GTN if both sides had the same degree of involvement. The target GTN also had to have an unaffected matrix, lunula, and length of at least 2 mm from the proximal nail fold; a thickness of at most 3 mm; and evidence of growth. Other inclusion criteria included age of at least 18 years, English literacy, not pregnant or lactating, and ability to participate in the study for one year. At the screening visit, written consent was obtained, after which the selected GTN(s) underwent clinical and OCT imaging and evaluation prior to histopathologic diagnosis with periodic acid-Schiff (PAS) staining. Subjects with at least one PAS-positive GTN qualified to continue on with the study. Thirty-four subjects were included in the study. Study inclusion and exclusion criteria were largely based on those in a previous clinical control trial for topical efinaconazole.5 Exclusion criteria included current or past history of immunosuppression, systemic or prescription topical antifungal therapy use within six months prior to screening, use of over-the-counter topical antifungal therapy or an investigational drug within thirty days prior to screening, toenail infection by an organism other than a dermatophyte, severe plantar (moccasin) tinea pedis infection on the target foot that would require systemic therapy, any pathology or surgery that might have caused toenail abnormalities that would interfere with evaluation, and an unwillingness to use an approved method of birth control during the study period (among fertile menstruating women).
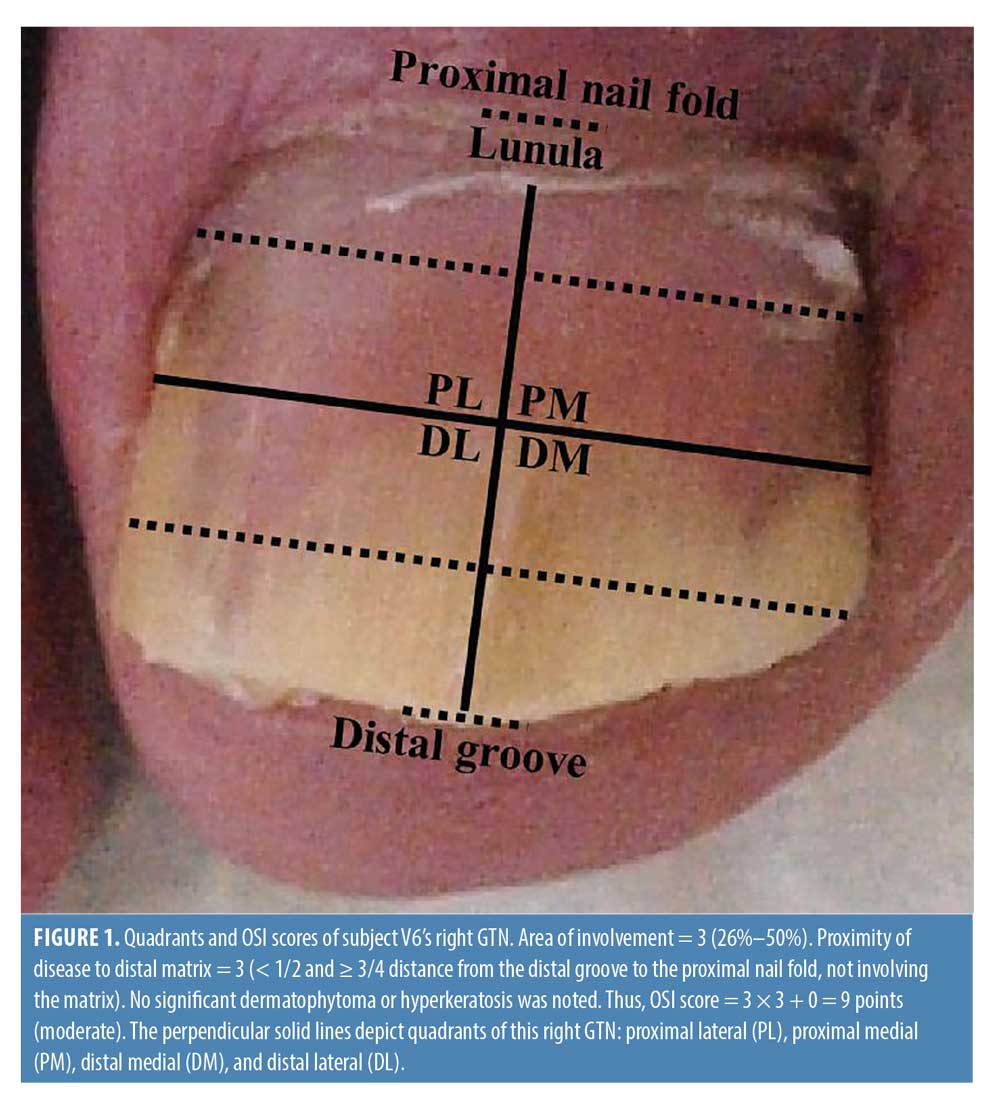
On study Day 1, which occurred within three weeks of the screening visit, target GTNs were photographed, scored using the Onychomycosis Severity Index (OSI) developed by Carney et al. (Figure 3),10 and scanned using OCT. On study Day 1, subjects commenced treatment with efinaconazole topical solution 10% applied once daily for 48 weeks. Eight follow-up visits occurred; the first four were every four weeks (Weeks 0, 4, 8, and 12), and the remaining four were every nine weeks (Weeks 21, 30, 39, and 48) (Figure 1). Clinical photography and OCT scanning of the target GTNs were performed at Weeks 0, 4, 8, 12, and 48 to monitor disease severity and visualize mycological pathology. At Weeks 21, 30, and 39, subjects returned to track medication usage, report any adverse events, and receive the next dose. At the final visit (Week 48), clinical and OCT images were taken and histopathologic specimens were collected for final PAS staining.
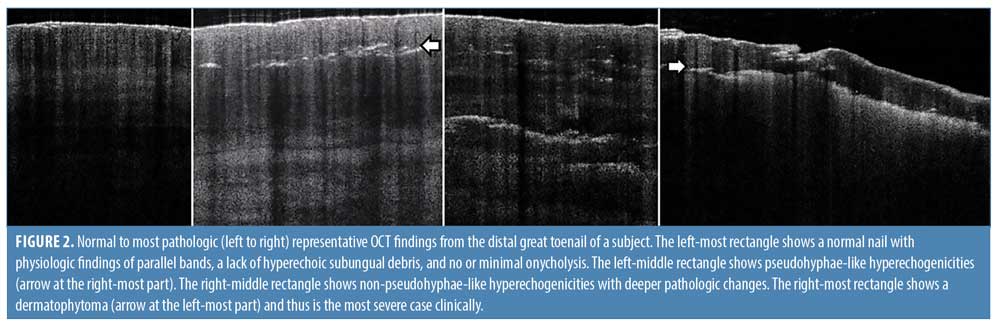
Frequency domain (FD-) OCT (VivoSight; Michelson Diagnostics Ltd., Maidstone, England) was used, which has a wavelength of 930 to 1,300 nm, penetration depth of 1.5 to 2 mm, lateral resolution of 7.5 to 15 um, axial resolution of 5 to 10 um, and field of view of 6 × 6 mm.11 Scans were taken of each quadrant (proximal lateral, distal lateral, distal medial, and proximal medial quadrants) at each visit, for a total of 680 scans included in the study (Figure 1). After training with a dermatologist with more than ten years of experience interpreting OCT scans, one researcher (C. X. C.) evaluated the scans for the following three findings suggestive of fungus in the nail plate: pseudohyphae-like hyperechogenicities in the distal nail plate, non-pseudohyphae-like hyperechogenicities in the distal nail plate, and dermatophytoma in the distal nail (Figure 2).

Our primary outcome was the association between OCT findings and final PAS result within twelve weeks of topical efinaconazole treatment. The secondary outcome was a comparison of the ability of OCT data with and without pairing with OSI data to predict the final PAS result also within the same time frame. Finally, the tertiary outcome was the complete cure rate, which was defined as an OSI score of zero plus mycological cure (negative PAS stain).
McNemar and chi-squared tests were used to compare clearance rates. Fisher’s exact test was used to describe the univariate relationship between six predetermined bivariate OCT variables and the bivariate final PAS result. SAS 9.4 (SAS Institute, Cary, NC, USA) was used to run regression models. A series of logistic regression models estimated the positive predictive value (PPV) and negative predictive value (NPV) of OCT for the PAS result at Week 48 as well as the odds ratio of the OSI score to predict the same outcome. The c-statistic was used to represent the area under a receiver operating characteristic plot and could vary from 0.5 (no utility) to 1.0 (perfect utility). Likelihood ratio tests of association are reported. Multiple regression models were used to describe the relationship between OCT and OSI results and the PAS result at Week 48. Significance was set at p<0.05.
Results
Thirty-four subjects were enrolled from the VA (n = 17) and Mount Sinai (n = 17). The mean patient age was 56 years at enrollment (51 among patients from the VA, 61 among patients from Mount Sinai). They were evenly split by sex (men totaled 53% of the population overall, 88% of the VA population, and 18% of the Mount Sinai population) and were of a variety of races, including white (35%), black (32%), Latino/Hispanic (18%), Asian (6%), and other/unknown (9%).
Our complete cure rate of 18% and mycological cure rate of 56% were statistically similar to the 14% and 54% rates of a previous clinical trial5 (both chi-square p greater or equal to 0.05) and were unlikely due to chance (both McNemar two-tailed p<0.05).
The analysis of OCT scans focused on three findings suggestive of past or present fungus in the nail plate (Figure 2). Pseudohyphae-like hyperechogenicities in the distal nail plate often appear as poorly-defined, hyperechoic, linear streaks that lie parallel to the nail surface, suggesting tracks left over from fungus previously present in that location.8 Non-pseudohyphae-like hyperechogenicities in the distal nail plate appear as multiple poorly-defined white structures and likely represent distortions left over from fungus previously present in that location. Finally, a dermatophytoma appears as a homogenous hyperechogenic area, with the most hyperechoic part being a well-defined border along the inferior edge of the nail plate,12 and correlate with the presence of fungus in that location.13
We found four distal nail OCT variables by Week 12 to be independently associated with positive PAS staining at Week 48 as follows: the presence of any of the three findings at Week 4 or 8 or 12 (n = 12, 13, and 11; Fisher’s two-tailed p = 0.011, 0.004, 0.003, respectively) or a lack of clearance of the three findings by Week 12 (n = 7; p = 0.028). The absence of any of the three findings by Week 12 (n = 14) was associated with negative PAS staining at Week 48 (p = 0.005). In contrast, clearance of the three findings by Week 12 was not significantly associated with the PAS result at Week 48 (p = 0.718).
For the PAS result at Week 48, the PPV and NPV of OCT with or without any of the three findings (“OCT+” or “OCTc”, respectively) were calculated (Table 1). The PPV increased from 0.60 [95% confidence interval (CI): 0.35 to 0.81] to 0.83 (95% CI: 0.52–0.96) from Week 0 to 12, and the NPV increased from 0.68 (95% CI: 0.45–0.85) to 0.77 (95% CI: 0.56–0.90) from Week 0 to 12. Meanwhile, the p-value decreased from 0.096 to < 0.001 from Week 0 to 12, and the c-statistic increased from 0.64 to 0.78 from Week 0 to 12.
A model combining OCT+/– and OSI severity at Week 12 was significantly associated with the PAS result at week 48 (c = 0.85; p < 0.001). This model was not statistically superior to a model containing only OCT, but was statistically superior to a model containing only OSI (p = 0.008). There were no statistically significant interactions between predictors in the multiple regression models.
For the PAS result at Week 48, the PPV and NPV of OCT+/– were recalculated for the subset of patients who had mild or moderate OSI scores at Week 0 (n = 23) (Table 1). Here, the PPV increased from 0.33 (95% CI: 0.08–0.73) at Week 0 to 0.67 (95% CI: 0.27–0.92) at both Weeks 8 and 12, while the NPV increased from 0.71 (95% CI: 0.46–0.87) at Week 0 to 0.82 (95% CI: 0.57–0.94) at both Weeks 8 and 12. The p-value also decreased from 0.858 at Week 0 to 0.029 at both Weeks 8 and 12, and the c-statistic increased from 0.52 at Week 0 to 0.72 at both Weeks 8 and 12.
In contrast, no significant association was found between OSI and treatment outcome for the subset of patients who had mild or moderate OSI scores at Week 0 (Table 2).

For the subset of patients who had mild or moderate OSI scores at Week 0 (n = 23), a model combining OCT+/– and OSI severity at Week 12 was significantly associated with the PAS staining result at week 48 (c = 0.86; p = 0.013). This model was not statistically superior to a model containing only OCT data from Week 12. Similar models for Weeks 4 and 8 yielded c-statistic values of 0.79 and 0.80, respectively. There were no statistically significant interactions between predictors in the multiple regression models.
Discussion
OCT findings were significantly associated with treatment outcome, the latter determined by PAS, one of the most specific methods of diagnosis.9 Having any of the three key OCT findings consistent with a history of fungus in the nail plate (i.e., pseudohyphae-like hyperechogenicities in the distal nail plate, non-pseudohyphae-like hyperechogenicities in the distal nail plate, and dermatophytoma in the distal nail) (Figure 2) by Week 12 was associated with a positive final PAS stain. Eighty-three percent of subjects who were OCT+ at Week 12 had a positive PAS stain at Week 48. On the other hand, the absence of all three OCT findings by Week 12 was associated with a negative final PAS stain. Notably, some of these cases may have had falsely positive initial PAS staining results. Eighty-two percent of subjects who had mild or moderate DLSO at week 0 and who were OCT– at Week 8 had a negative final PAS stain.
In contrast, OSI score was not associated with PAS stain results at week 48 in patients who had mild or moderate DLSO at Week 0. Adding OSI data to a predictive model that included OCT data did not improve the model. Other research has found that nonimaging variables, including sex, disease duration, and involvement of other toenails, are not predictive of clearance.5 Early mycological cure may be associated with a greater chance of clearance but would not be evaluated until six months into treatment.14
Our results are clinically important given the high financial and temporal cost of topical efinaconazole. One regimen of the treatment costs $4,020 per GTN over 334 days of application, based on each GTN needing two drops daily, and 20 drops (1 mL) costing $120. That represents a significant commitment for many patients, especially if they are unable to predict treatment outcome using a drug that has a mycological cure rate of only 54%.5 Our results suggest a pivotal role for noninvasive imaging in alleviating that issue.
Alternatives to efinaconazole for mild-to-moderate DLSO include oral terbinafine, which has a lower cost but can cause various side effects, including drug–drug reactions as a CYP2D6 inhibitor, headache, dermatitis, gastrointestinal distress, taste disturbances, liver enzyme abnormalities, drug eruptions such as Stevens–Johnson syndrome, and thrombotic microangiopathy. In contrast, topical efinaconazole has no contraindications or systemic side effects.
We recommend that a FD-OCT scan of the distal nail plate focusing on the three findings utilized in this study be obtained at two months into the treatment course to inform shared decision-making regarding whether to continue topical efinaconazole for nine additional months (Figure 3). This recommendation is most applicable to the on-label disease population of mild-to-moderate DLSO at treatment initiation. Depending on the OCT findings at eight weeks, the patient would have up to a 77% chance of mycological nonclearance or up to an 82% chance of mycological clearance by Week 48. These predictive values are both significantly higher than the current average mycological cure rate of only 54%.5
Limitations. Limitations of this study include a sample size of 34 patients that was powered to detect only large differences. Significant differences were detected, and future prospective studies with higher power are recommended. Another limitation is the lack of national standardization for interpreting OCT scans of nails. To avoid interrater variability, only one researcher was responsible for reading the scans, after receiving training from a dermatologist experienced in using the technology. Greater utilization of OCT by dermatologists may lead to more standardized training, evaluation, and reimbursement within the field. For providers, the cost of a Vivosight machine is on the lower end of lasers, varies with location and usage, and is more favorable to lease than other lasers. For patients, OCT is expected to become reimbursed at a level similar to other diagnostic modalities, such as reflectance confocal microscopy and histology.
Conclusion
Our study is the first to show OCT’s ability to evaluate onychomycosis outcome with use of topical efinaconazole in a manner earlier and more reliably than nonimaging variables can achieve. Incorporating this technology into shared decision-making could improve clinical care and decrease the medical and financial burdens of onychomycosis for millions of patients.
Acknowledgment
Jeremy Weedon, PhD, is from SUNY Downstate Health Sciences University in New York, New York, and provided invaluable statistical guidance and analysis.
References
- Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population—a literature study. J Eur Acad Dermatol Venereol. 2014;28(11):1480–1491.
- Rosen T, Friedlander SF, Kircik L, et al. Onychomycosis: epidemiology, diagnosis, and treatment in a changing landscape. J Drugs Dermatol. 2015;14(3):223–233.
- Lipner SR, Scher RK. Onychomycosis: clinical overview and diagnosis. J Am Acad Dermatol. 2019;80(4):835–851.
- GoodRx. Efinaconazole. Available at: https://www.goodrx.com/efinaconazole?dosage=8ml-of-10%&form=bottle-of-topical-solution&label_override=Jublia&quantity=1. Accessed January 27, 2020.
- Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68(4):600–608.
- Velasquez-Agudelo V, Cardona-Arias JA. Meta-analysis of the utility of culture, biopsy, and direct KOH examination for the diagnosis of onychomycosis. BMC Infect Dis. 2017;17(1):166.
- United States Food and Drug Administration. Re: K153283 Trade/Device Name: VivoSight Dx Topical OCT System. To Michelson Diagnostics Ltd. From Ronk CJ, Ashar BS. 2016 Aug 11. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf15/K153283.pdf. Accessed January 27, 2020.
- Abuzahra F, Spöler F, Först M, et al. Pilot study: optical coherence tomography as a non-invasive diagnostic perspective for real time visualisation of onychomycosis. Mycoses. 2010;53(4):334–339.
- Rothmund G, Sattler EC, Kaestle R, et al. Confocal laser scanning microscopy as a new valuable tool in the diagnosis of onychomycosis—comparison of six diagnostic methods. Mycoses. 2013;56(1):47–55.
- Carney C, Tosti A, Daniel R, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011;147(11):1277–1282.
- Schwartz M, Levine A, Markowitz O. Optical coherence tomography in dermatology. Cutis. 2017;100(3):163–166.
- Verne SH, Chen L, Shah V, et al. Optical coherence tomography features of dermatophytoma. JAMA Dermatol. 2018;154(2):225–227.
- Burkhart CN, Burkhart CG, Gupta AK. Dermatophytoma: recalcitrance to treatment because of existence of fungal biofilm. J Am Acad Dermatol. 2002;47(4):629–631.
- Jellinek NJ, Korotzer A. Prognostic factors for complete cure following treatment of mild and moderate toenail onychomycosis with efinaconazole topical solution 10. J Drugs Dermatol. 2015;14(8):871–875.

