 J Clin Aesthet Dermatol. 2023;16(1):41-46.
J Clin Aesthet Dermatol. 2023;16(1):41-46.
by Khaled Mohey El-Din Monib, MD; Neveen Emad Sorour, MD;
Naglaa Fathy Alhusseini, MD; Asmaa Gaber Abdou, MD; Amal Yousif, MD; and
Rehab Mohammed Salem, MD
Drs. Monib and Sorour are Professors of Dermatology and Andrology, Faculty of Medicine at Benha University in Benha, Egypt. Dr. Alhusseini is a Professor of Medical Biochemistry and Molecular Biology, Faculty of Medicine at Benha University in Benha, Egypt. Dr. Abdou is a Professor of Pathology, Faculty of Medicine at Menoufia University, Egypt. Dr. Yousif is a Lecturer of Dermatology and Andrology, Faculty of Medicine at Benha University in Benha, Egypt. Dr. Salem is an Assistant Professor of Dermatology and Andrology, Faculty of Medicine at Benha University in Benha, Egypt.
ABSTRACT: Background. The search for objective factors that help in predicting the response of vitiligo treatment is very important.
Objective. We sought to evaluate the effect of NB-UVB phototherapy on both the alpha melanocyte stimulating hormone-microphthalmia-associated transcription factor (α-MSH-MIFT) axis, and isocitrate dehydrogenase 2 (IDH2) in non-segmental vitiligo (NSV).
Methods. This prospective clinical trial included 50 NSV patients and 50 healthy control subjects. α-MSH tissue levels as well as MITF and IDH2 immunostaining were assessed in normal and vitiliginous skin biopsies before treatment and then in the repigmented areas following 24 NB-UVB phototherapy treatment sessions using ELISA technique and immunohistochemical study, respectively.
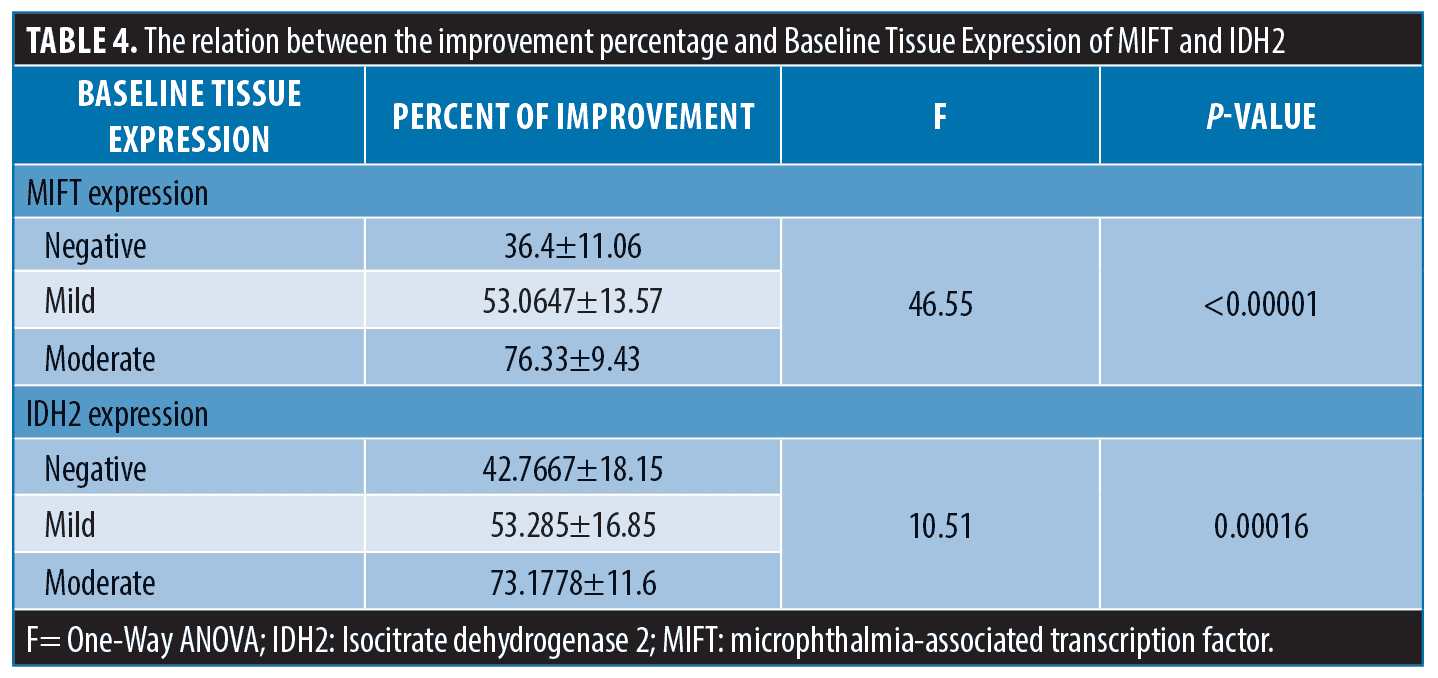
Results. There was a significant negative correlation between baseline VASI scores and the tissue levels of α-MSH (p=0.006) and the expression of both MITF (p<0.00001) and IDH-2 (p= 0.001). The mean α-MSH tissue levels increased significantly after treatment (p<0.001). Tissue expression of both MTIF and IDH-2 was significantly upregulated following treatment (P-value <0.001). The percentage of improvement showed a significant positive correlation with the studied markers (p<0.00001).
Conclusion. α-MSH- MIFT axis and the antioxidant protein IDH2 are promising objective markers of non-segmental vitiligo severity, and are suggested as predictors of vitiligo response to treatment.
Vitiligo is a chronic autoimmune depigmenting skin disease resulting from acquired loss of melanocytes. It is usually associated with a negative impact on the quality of life and marked psychosocial distress.1
The exact pathogenesis of vitiligo is not fully understood and many hypotheses have been proposed to explain the etiology of melanocytes loss in the vitiliginous patches. Among the suggested theories for vitiligo development; the toxic effects of the oxidative stress on melanocytes is one of the highly accepted ones. There are multiple natural antioxidant substances in the body. These natural antioxidants can clear the harmful oxidative products which are produced in the body during many processes including melanogenesis. Disturbance in the natural oxidant- antioxidant balance may lead to melanocytes damage and development of vitiligo.2 Isocitrate dehydrogenase 2 (IDH2) is a mitochondrial enzyme which fights reactive oxygen species (ROS) as one of the endogenous antioxidant proteins by converting NADP+ to NADPH with a subsequent reduction of oxidized glutathione into reduced glutathione.3
Melanogenesis is a physiological process that requires an interaction between the melanocytes and its neighboring keratinocytes. Keratinocytes, upon UV exposure release a melanogenesis regulatory product called α-melanocyte-stimulating hormone (α-MSH), which binds to its receptor on the melanocyte cell surface [melanocortin 1 receptor (MC1R)]. The binding between this receptor with its ligand initiates an intracellular cascade of interactions the ends up with activation of the transcription of a variety of downstream targets, including microphthalmia-associated transcription factor (MITF).4 MIFT is involved in regulating the activity of several enzymes in the synthetic pathway of melanin such as tyrosinase, tyrosinase related protein 1, tyrosinase related protein 2 and melanocytic differentiation markers.5 MITF is also one of the melanocyte differentiation, proliferation, survival, and activity regulatory factors.6
The current study aimed to evaluate the effect of narrow band UVB (NB-UVB) phototherapy on both the α melanocyte stimulating hormone- microphthalmia-associated transcription factor (α-MSH-MIFT) axis, as well as the antioxidant protein IDH2 in vitiliginous patches in non-segmental vitiligo patients.
Methods
This prospective clinical trial was conducted on 50 patients with non-segmental vitiligo of different sex and age groups attending the out-patient clinic and the phototherapy unit of Dermatology and Andrology Department, Benha University Hospital in the period between April 2019 to January 2021. In addition, 50 apparently healthy control subjects were included as a control group.
The work started after obtaining the approval of the Department of Dermatology and Andrology and the Research Ethics Committee in Faculty of Medicine, Benha University (MD 13.3.2019). Informed consents were taken from all participants before involvement in the study.
Exclusion criteria. Patients with segmental vitiligo, any contraindication to phototherapy as photosensitive dermatoses (e.g., lupus erythematosus), and those who received local or systemic treatment for vitiligo two months before the start of the study were excluded from this work. Pregnancy, lactation, and other autoimmune diseases as diabetes mellitus and autoimmune thyroiditis were also among the exclusion criteria.
Methodology. Baseline Assessment.
All patients were subjected to history taking, and skin examination to detect the type and the distribution of vitiligo. The disease severity was evaluated using Vitiligo Area Severity Index (VASI).7 Vitiligo Disease Activity (VIDA) Score was used for evaluating vitiligo activity.8
Alpha melanocyte stimulating hormone (α-MSH) tissue levels as well as microphthalmia-associated transcription factor (MITF) and isocitrate dehydrogenase 2 (IDH2) immunostaining were assessed in normal and vitiliginous skin biopsies before treatment using ELISA technique and immunohistochemical staining, respectively.
Treatment protocol. Patients were treated by narrow band UVB (NB UVB) (Herbert Waldmann Gmbh & Co KG, made in Germany) for 24 sessions (3 sessions a week) according to Khanna and Khandpur9 protocol.
Re-evaluation. After the 24 treatment sessions, all patients were evaluated again, clinically by VASI score and were underwent skin biopsy from repigmented areas for assessment of the changes in the studied markers (α-MSH, MITF, IDH2).
Tissue sampling. One target patch of vitiligo was selected in each patient. For each patient, two skin biopsies were performed from the target depigmented area at baseline prior to treatment. The second biopsies were obtained from the same lesion of interest after the 24 treatment sessions. Each biopsy site was locally anesthetized with 2% xylocaine without adrenaline, and samples were obtained using a 4mm punch biopsy.
For each patient, two skin biopsy specimens were taken, which included the hypodermis, one specimen was fixed immediately in 10% formalin and sent to Pathology Department and Faculty of Medicine at Menoufia University. The other tissue sample was preserved in Eppendorf tube at -80°C and sent to the Biochemistry Department and the Faculty of Medicine at Benha University.
Immunohistochemical study. Formalin preserved samples were submitted to routine tissue processing ending with paraffin embedded blocks formation. Two, 5 micron cut sections had been done. One section was stained with hematoxylin and eosin for evaluation of histopathological changes. The other section was cut on poly L lysine coated slides for immunohistochemical staining. The method used in immunostaining was streptavidin-biotin-amplified system. The antibodies were rabbit polyclonal antibody raised against synthesized peptide of human IDH2 (P48735, 51kD, diluted as 1/75, the positive control was human cervical cancer tissue; SRB Laboratories, Catlog No.: 201r-5702) and MITF (O75030, 59kD, dilutes as 1/50, the positive control was human thyroid cancer tissue) which react against human, mouse, and rat tissues. Each vial contains 0.1ml (100μg/100μl). DAB was used as a chromogenic substrate and Mayer’s hematoxylin was used as a counterstain.
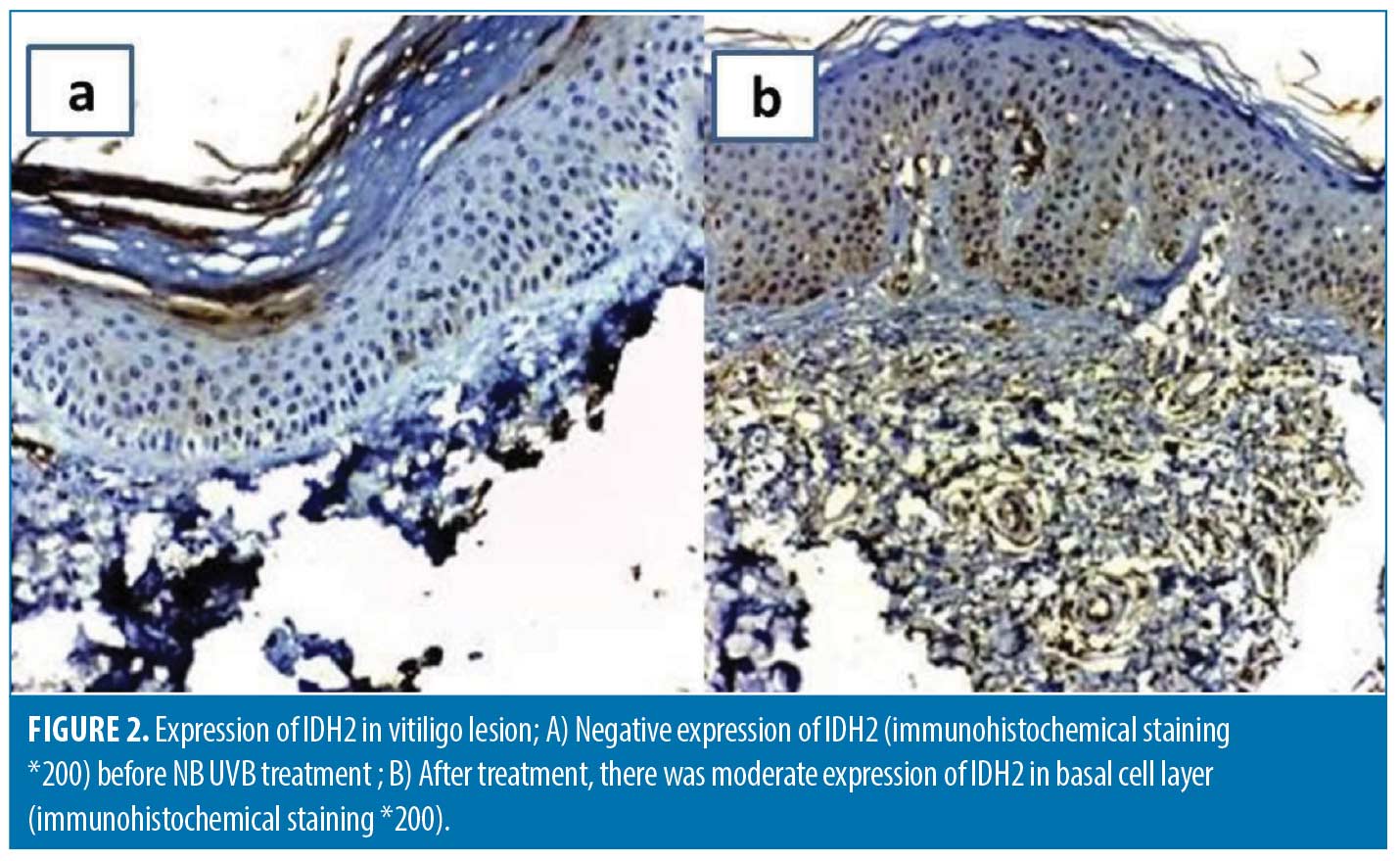
Cytoplasmic expression of IDH2 and MITF in any number of keratinocytes was designated as positive expression while the intensity of expression was assessed subjectively according to depth of brown color into mild (+), moderate (++), and strong (+++).
ELISA for tissue level of α-MSH. Tissue samples were preserved in Eppendorf tubes at -80°C then they were submitted to the ELISA plate assay to detect the level of tissue α-MSH using a monoclonal antibody specific to α-MSH. The minimum detectable dose of α-MSH is typically less than 6.43pg/mL (Human α-MSH ELISA KIT; Shanghai Sunred Biological Technology, China).
Statistical methods. Data management and statistical analysis were done using SPSS version 25. (IBM, Armonk, New York, United States). Quantitative data were assessed for normality using Kolmogorov–Smirnov test and direct data visualization methods. Numerical data were summarized as means and standard deviations or medians and ranges. Categorical data were summarized as numbers and percentages. Suitable tests were selected according to the nature of data to be analyzed. p-values less than 0.05 were considered significant.
Results
There was insignificant difference between patients and control subjects regarding age (p= 0.08) and sex (p= 0.52) (Table 1).
The median disease duration was six years and ranged from four months to 20 years. About one-third of the patients (15 patients) showed positive family history (30.0%). The most frequent exacerbating factor was psychological stress in 30 patients (60.0%), followed by physical trauma in 12 patients (24.0%) and sun exposure in eight patients (16.0%).
Baseline assessment. The baseline evaluation of vitiligo severity showed that the median baseline VASI score was 7.5 (range=1- 23.9). Regarding the disease activity; three patients (6%) stable for at least one year (VIDA 0); five patients (10%) showed activity in the past year (VIDA +1), and 11 patients (22%) reported active disease in the past six months (VIDA +2). Activity in the past three months was reported in 19 patients (38%) (VIDA +3) and the disease was active in the past six weeks (VIDA +4) in 12 patients (24%).
The control samples showed significantly higher levels of α–MSH and significantly stronger expression for MIFT and IDH-2 compared to vitiliginous skin (p<0.0001) (Table 1).
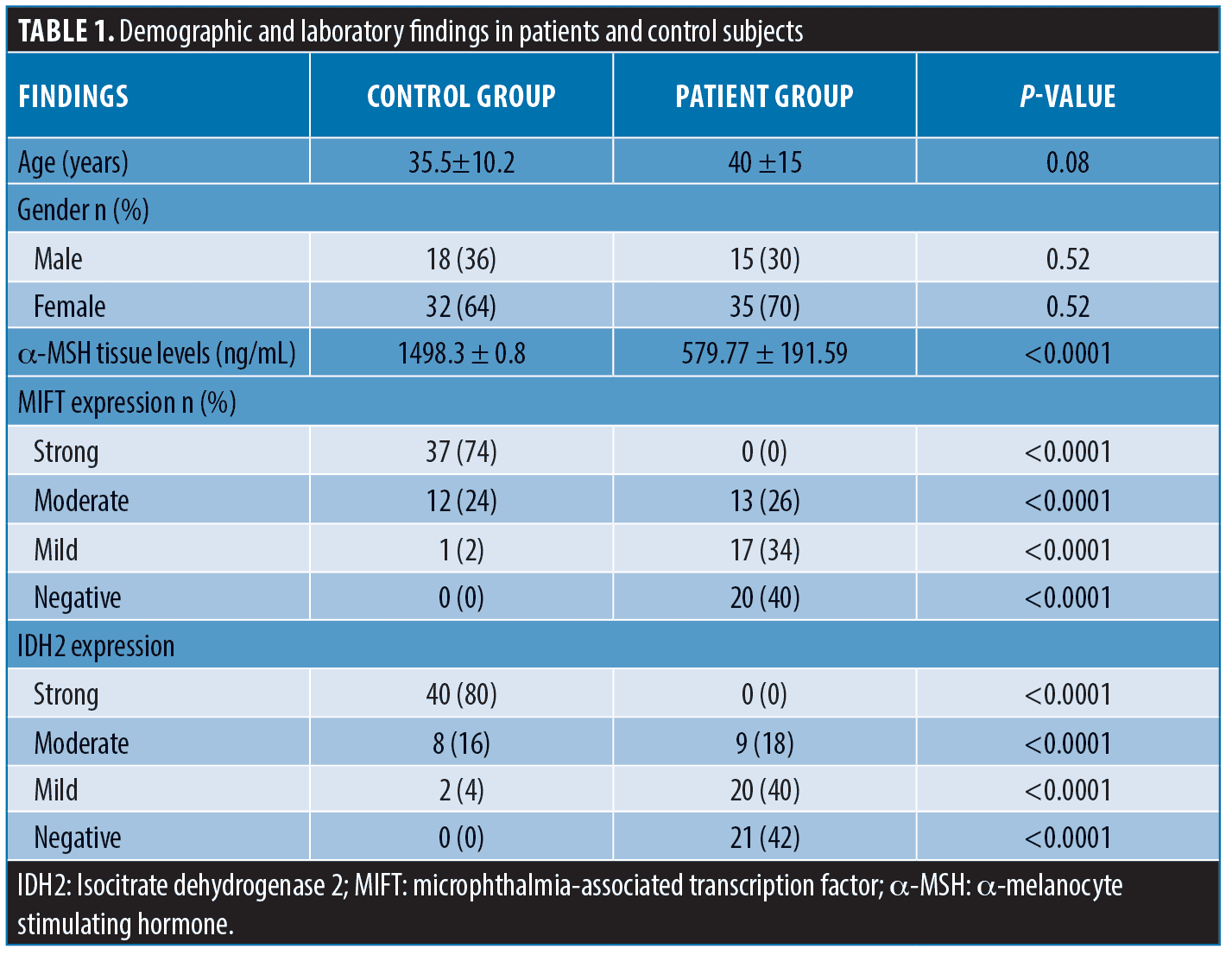
Before treatment, MITF expression in vitiliginous specimens was negative in 20 patients (40.0%), mild in 17 patients (34.0%), and moderate in 13 patients (26.0%). IDH-2 expression was negative in 21 cases (42%), mild in 20 cases (40%) and moderate in nine cases (18%) (Table 2).
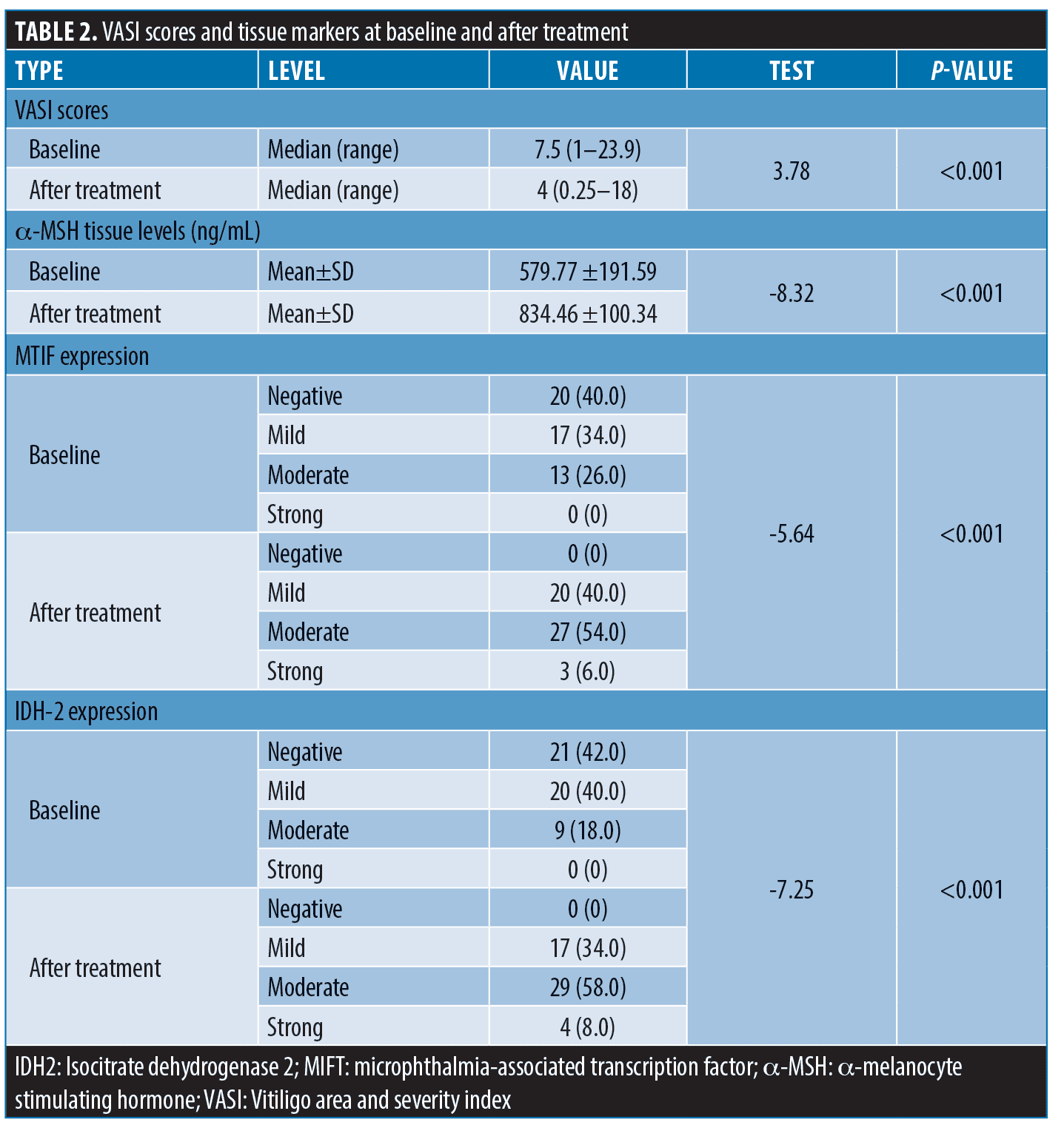
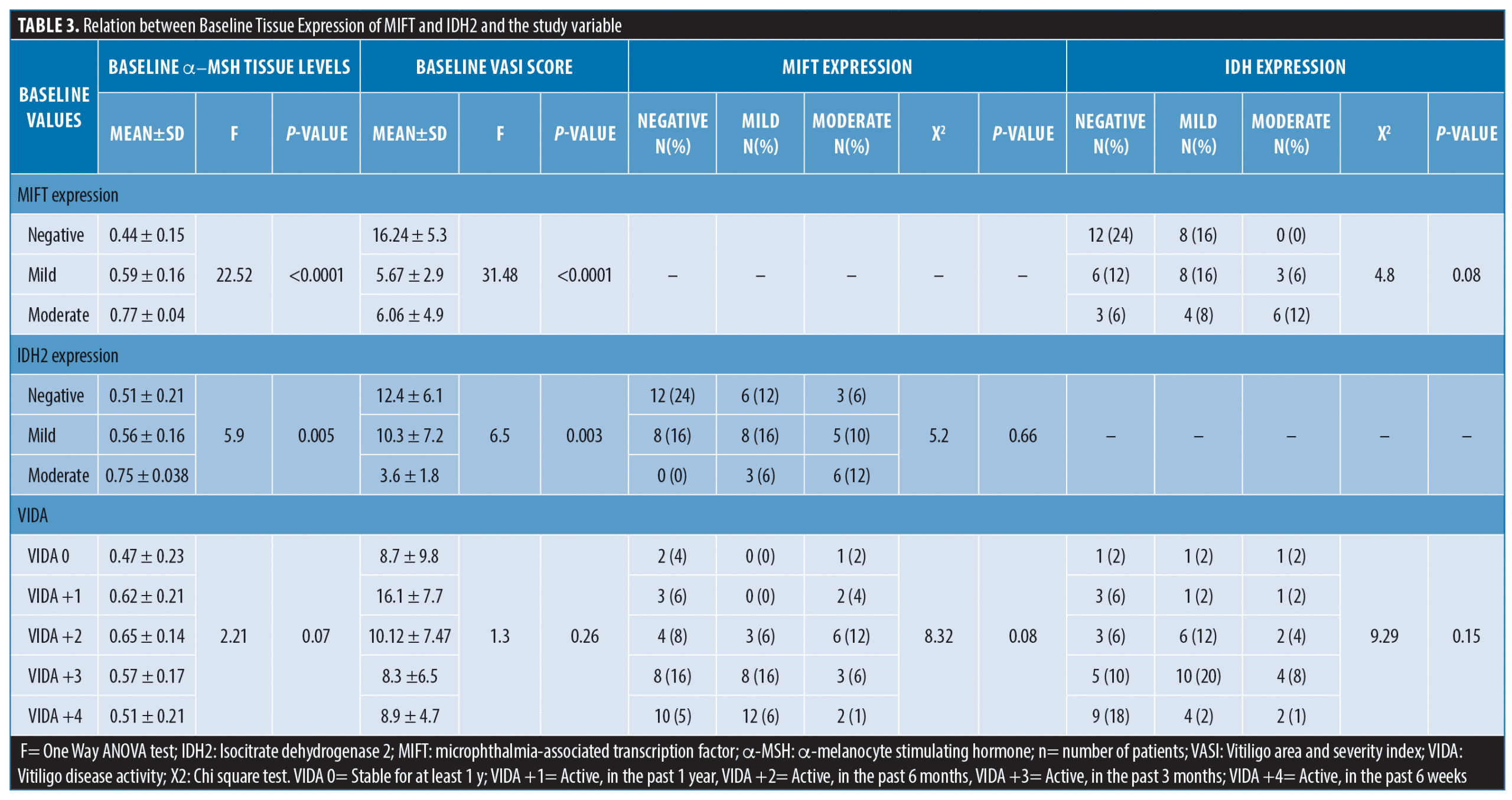
The mean α–MSH tissue levels were 579.77 ±191.59ng/mL. There was a significant negative correlation between the tissue levels of α–MSH and the disease severity (baseline VASI score) (r=-0.3891, p=0.006), but not the disease activity (VIDA score) (f=2.21, p=0.07). Higher tissue levels of α–MSH were also associated with more intense expression of MIFT and IDH2 (p<0.0001 and 0.005; respectively). The absence of MIFT or IDH2 expression was associated with a significantly higher baseline VASI scores (Table 3).
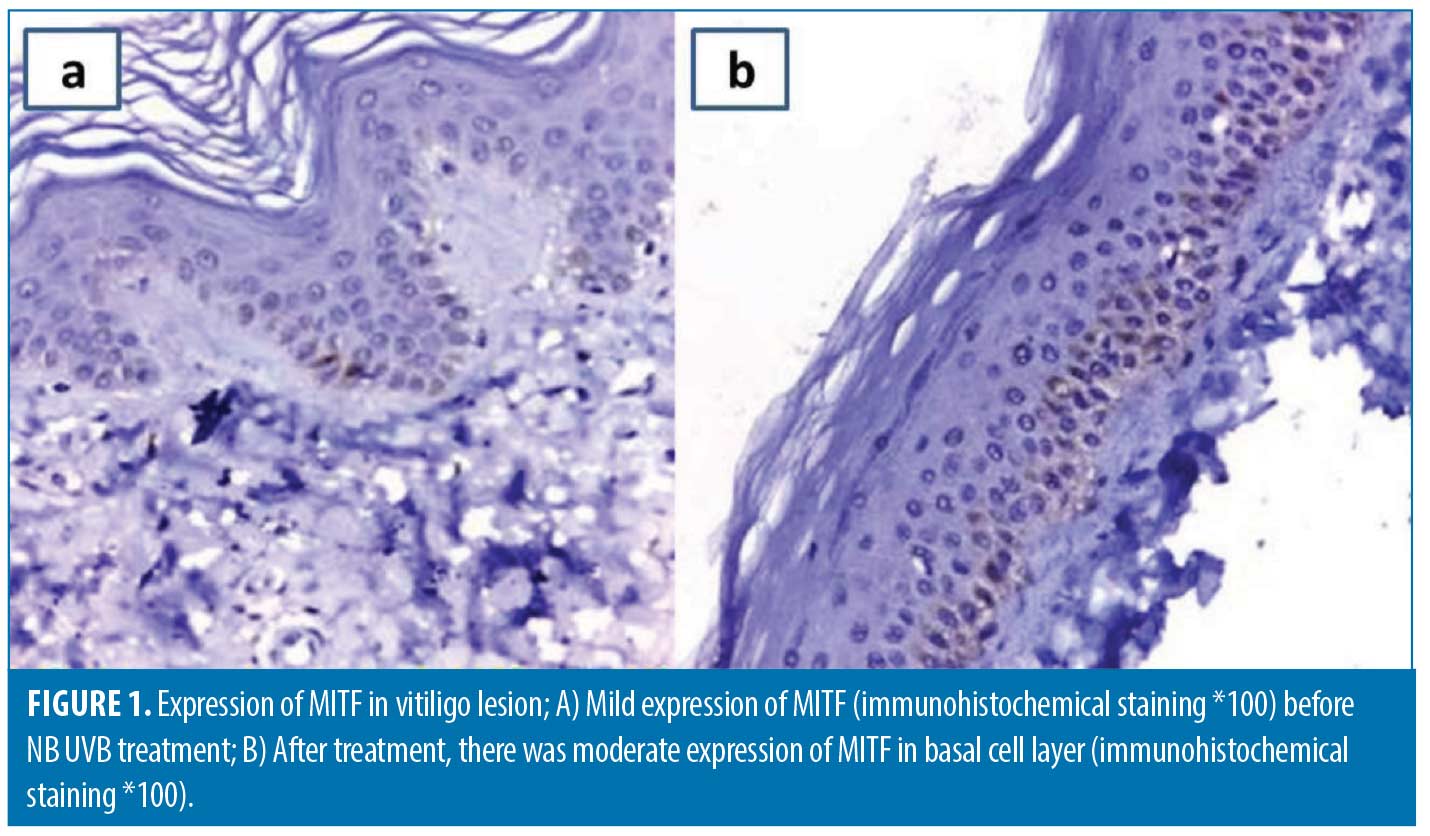
Re-assessment after NB UVB treatment.The degree of clinical improvement was assessed by evaluating the percentage of VASI score changes. The median VASI score significantly declined at the end of treatment to four compared to 7.5 at baseline (p<0.001) and the improvement percentage ranged from 16.7 to 87.5 percent. The mean α–MSH tissue levels significantly increased after treatment (p-value <0.001) (Table 2). Tissue expression of both MTIF (Figure 1) and IDH-2 (Figure 2) was significantly upregulated following treatment (p<0.001). The percentage of improvement in VASI score showed a significant positive correlation with baseline α–MSH tissue levels (r= 0.7769, p<0.00001). The percentage of improvement was significantly higher in patients with more intense baseline MIFT and IDH2 expression (p<0.0001 and 0.00016; respectively) (Table 4).
Discussion
Examination of the current baseline skin biopsies revealed a significant association between elevated α-MSH tissue levels and MITF and IDH2 stronger expression. In fact, there is a relation between MSH activity and the level of oxidative stress. Kadekaro et al10 suggested that besides the major role of α-MSH in regulating melanogenesis, it has also antioxidant properties. Spencer and Schallreuter11 found that the accumulated H2O2 in vitiligo epidermis exerts a suppressing effect on α-MSH peptide, and the NB-UVB correction of the oxidant antioxidant imbalance in vitiligo patches leads to enhancement of α-MSH peptide tissue expression. This could provide a logic explanation of the significant relation between α-MSH/MITF axis and IDH2.
The current study showed that there is a significant relation between α-MSH/MITF axis and IDH2 and disease severity (VASI scores). The relationship between vitiligo severity and the degree of oxidative stress is controversial. Praharsini et al12 found that serum malondialdehyde level correlates positively with vitiligo severity (VASI scores). While the studied oxidative stress parameters (advanced oxidation protein products, prooxidant-antioxidant balance, and ferric-reducing antioxidant power) in Guntas et al13 study did not correlate with VASI scores. The discrepancy between studies may be attributed to the different studied oxidative stress parameters.
To the best of our knowledge, the relationship between the currently studies axis and the disease severity was not previously evaluated. UVB-induced α-MSH/MC1R/m-MITF axis is an eminent pathway in controlling the process of melanogenesis and skin pigmentation.14 Therefore, the relation between vitiligo extent and the activity of this axis seems to be logic.
After NB-UVB phototherapy treatment course, the current non-segmental vitiligo patients showed a significant clinical improvement (significant reduction in VASI scores). This clinical improvement was associated with a significant elevation in α-MSH levels measured by ELISA, and MIFT immunoreactivity. MSH/MC1R is involved in the activation, migration, and differentiation of the follicular melanocyte stem cells and melanoblasts leading to perifollicular repigmentation in NB-UVB phototherapy treated vitiliginous patches.14
The results of the present study showed that IDH2 immunohistochemical expression was significantly upregulated in areas of perifollicular repigmentation after NB-UVB phototherapy. As far as we know, the effect of NB-UVB therapy on IDH2 enzyme in vitiligo patients was not previously investigated, while its activating effect on other antioxidant enzymes was previously proposed. Karsli et al15 found that NB-UVB phototherapy was associated with a significant increase in glutathione peroxidase (GSH-Px) levels, compared to the baseline, in patients with vitiligo. Garg et al16 found that NB-UVB phototherapy treatment also improves the catalase and superoxide dismutase activity in stable vitiligo patients when compared to their baseline activity.
The antioxidant enzyme IDH2 was not previously investigated in vitiligo, however its relationship to skin pigmentation was evaluated by Park et al3 who conducted their study on white rats. They confirmed that silencing of IDH2 resulted in an increase in the levels of both oxidative DNA damage and melanogenesis signals such as α-MSH and POMC. The expression level of α-MSH was evaluated with ELISA and immunoblot analyses. This in vitro study demonstrated that the downregulation of IDH2 expression in keratinocytes results in the generation and secretion of α-MSH via p53 apoptosis signal pathways. The contrast between Park et al3 study and the current study can be explained by the difference in both studies samples. Park et al3 conducted their study on healthy white rats’ skin not vitiliginous skin, so there was no imbalance in the oxidant-antioxidant system, while the current study investigated IDH2 in vtitiligo skin where the oxidant-antioxidant system is already disturbed. It seems that more studies investigating the exact behavior of this enzyme in vitiligo are required.
The ability of NB-UVB to induce repigmentation of vitiliginous patches via stimulation of α-MSH/MC1R/m-MITF axis was previously proved by Spencer and Schallreuter11, Listiawan et al17 and Gauthier et al.18 Its ability also to reduce the oxidative stress by enhancing antioxidant enzymes was also previously proved by Karsli et al15 and Garg et al16 This proposes that the activation of α-MSH/MC1R/m-MITF axis and IDH2 enzyme in treated vitiligo patches to be a useful indicator of the efficiency of the used therapeutic option and to represent an objective proof of treatment success on the tissue level. However, as we know, the ability of the degree of this axis reactivity and this enzyme activity in the tissue to predict the response to treatment was not investigated in previous studies. In the current study, there was a significant positive correlation between the amount of α-MSH and the percentage of improvement (% of VASI scores reduction). Moreover, stronger MITF as well as IDH2 activity in the tissue were associated with significantly higher percentage of clinical improvement.
Considering the variable response of vitiliginous patients to the different available therapeutic lines, finding an objective predictive factor might be helpful in choosing the suitable treatment option and in discussing the anticipated prognosis of the condition with our patients. Previous works have proposed MiRNA 196a2C/T gene polymorphism, serum tyrosinase levels (19) and DEFB1 gene polymorphisms (20) as reliable predictors of vitiligo response to NB-UVB. The current findings could suggest the amount of α-MSH and the level of MITF as well as IDH2 expression in the tissue to be a promising predictors of vitiligo response to NB-UVB phototherapy. However, more studies are needed to find out more predictors.
References
- Morales-Sánchez MA, Vargas-Salinas M, Peralta-Pedrero ML, et al. Impact of Vitiligo on Quality of Life. Actas Dermosifiliogr. 2017 Sep;108(7):637–642.
- Chen J, Li S, Li C. Mechanisms of melanocyte death in vitiligo. Med Res Rev. 2021;41(2):1138–1166.
- Park JH, Ku HJ, Lee JH, et al. Idh2 Deficiency Exacerbates Acrolein-Induced Lung Injury through Mitochondrial Redox Environment Deterioration. Oxid Med Cell Longev. 2017;2017:1595103.
- García-Borrón JC, Abdel-Malek Z, Jiménez-Cervantes C. MC1R, the cAMP pathway, and the response to solar UV: extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014;27(5):699–720.
- Kawakami A, Fisher D. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab Invest. 2017; 97:649–656.
- Fang D, Setaluri V. Role of microphthalmia transcription factor in regulation of melanocyte differentiation marker TRP-1. Biochem Biophys Res Commun. 1999 Mar 24;256(3):657–663.
- Hamzavi I, Jain H, McLean D, et al. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: the Vitiligo Area Scoring Index. Arch Dermatol. 2004 Jun;140(6):677–683.
- Njoo MD, Das PK, Bos JD, et al. Association of the Koebner phenomenon with disease activity and therapeutic responsiveness in Vitiligo Vulgaris. Arch dermatol. 1999 ; 135:407–413.
- Khanna U, Khandpur S. What Is New in Narrow-Band Ultraviolet-B Therapy for Vitiligo? Indian Dermatol Online J. 2019 May-Jun;10(3):234–243.
- Kadekaro AL, Chen J, Yang J, et al. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol Cancer Res. 2012 Jun;10(6):778–786.
- Spencer JD, Schallreuter KU. Regulation of pigmentation in human epidermal melanocytes by functional high-affinity beta-melanocyte-stimulating hormone/melanocortin-4 receptor signaling. Endocrinology. 2009 Mar;150(3):1250–1258.
- Praharsini GAA, Wiraguna AAG, Batan PNW. The positive correlation between serum malondialdehyde levels with vitiligo severity and activity. BDV. 2019; 2(1):12–16.
- Guntas G, Engin B, Ekmekçi ÖB, et al. Evaluation of advanced oxidation protein products, prooxidant-antioxidant balance, and total antioxidant capacity in untreated vitiligo patients. Ann Dermatol. 2015; 27(2):178–183.
- Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp Dermatol. 2021;30(4):560–571.
- Karsli N, Akcali C, Ozgoztasi O, et al. Role of oxidative stress in the pathogenesis of vitiligo with special emphasis on the antioxidant action of narrowband ultraviolet B phototherapy. J Int Med Res. 2014 Jun;42(3):799–805.
- Garg K, Rathore B, Misra A, et al. Assessment of Oxidative Stress During Narrow Band UVB Phototherapy In Vitiligo Patients of North India. Int J Bioassays. 2014;3 (08):3231–3235
- Listiawan MY, Wardh PH, Astari L (2019): comparison amount of microphthalmia-associated transcription factor in vitiligo before and after narrowband- ultraviolet B therapy. Dermatology Reports. 11(1):8030.
- Gauthier Y, Almasi-Nasrabadi M, Cario-André M, et al. Tacrolimus (FK506) ointment combined with Nb-UVB could activate both hair follicle (HF) and dermal melanocyte precursors in vitiligo: the first histopathological and clinical study. Arch Dermatol Res. 2021;313(5):383–388.
- Monib KME, Sabry HH, Hussein MS, et al. Factors affecting vitiligo response to treatment: do MiRNA 196a2C/T gene polymorphism and serum tyrosinase levels have any role? J Dermatolog Treat. 2020:1–5.
- Salem RM, Abdelrahman AMN, Abd El-Kareem HM, et al. DEFB1 gene polymorphisms modify vitiligo extent and response to NB-UVB phototherapy. Dermatol Ther. 2021;34(3):e14921.

