J Clin Aesthet Dermatol. 2023;16(9):28–32.
J Clin Aesthet Dermatol. 2023;16(9):28–32.
by Pooja H. Rambhia, MD; Angel D. Pagan, BS; Karan Lal, DO, MS; and David Goldberg, MD, JD
Dr. Rambhia is with the Department of Dermatology at Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in New Hyde Park, New York. Mr. Pagan is with Ponce Health Sciences University School of Medicine in Ponce, Puerto Rico. Drs. Lal and Goldberg are with the Skin Laser and Surgery Specialists Division of Schweiger Dermatology in New York, New York. Addtionally, Dr. Goldenberg is with the Department of Dermatology at Icahn School of Medicine at Mount Sinai in New York, New York.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors declare no relevant conflicts of interest for this study.
ABSTRACT: Background. Hypopigmented scars are challenging to treat due to a lack of effective treatments and often transient results. Recent reports suggest that prostaglandin analog-induced hyperpigmentation may have favorable dermatological applications.
Objective. Analyze previous studies involving the use of prostaglandin analogs in the treatment of hypopigmented scars.
Methods. PubMed/Medline was queried through 10/01/2022 with the following search terms: (bimatoprost AND scar), (latanoprost AND scar), (travoprost AND scar), (prostaglandin analogs AND hypopigmented scars), (PGF2alpha AND hyperpigmentation), (prostaglandin analogs AND hyperpigmentation).
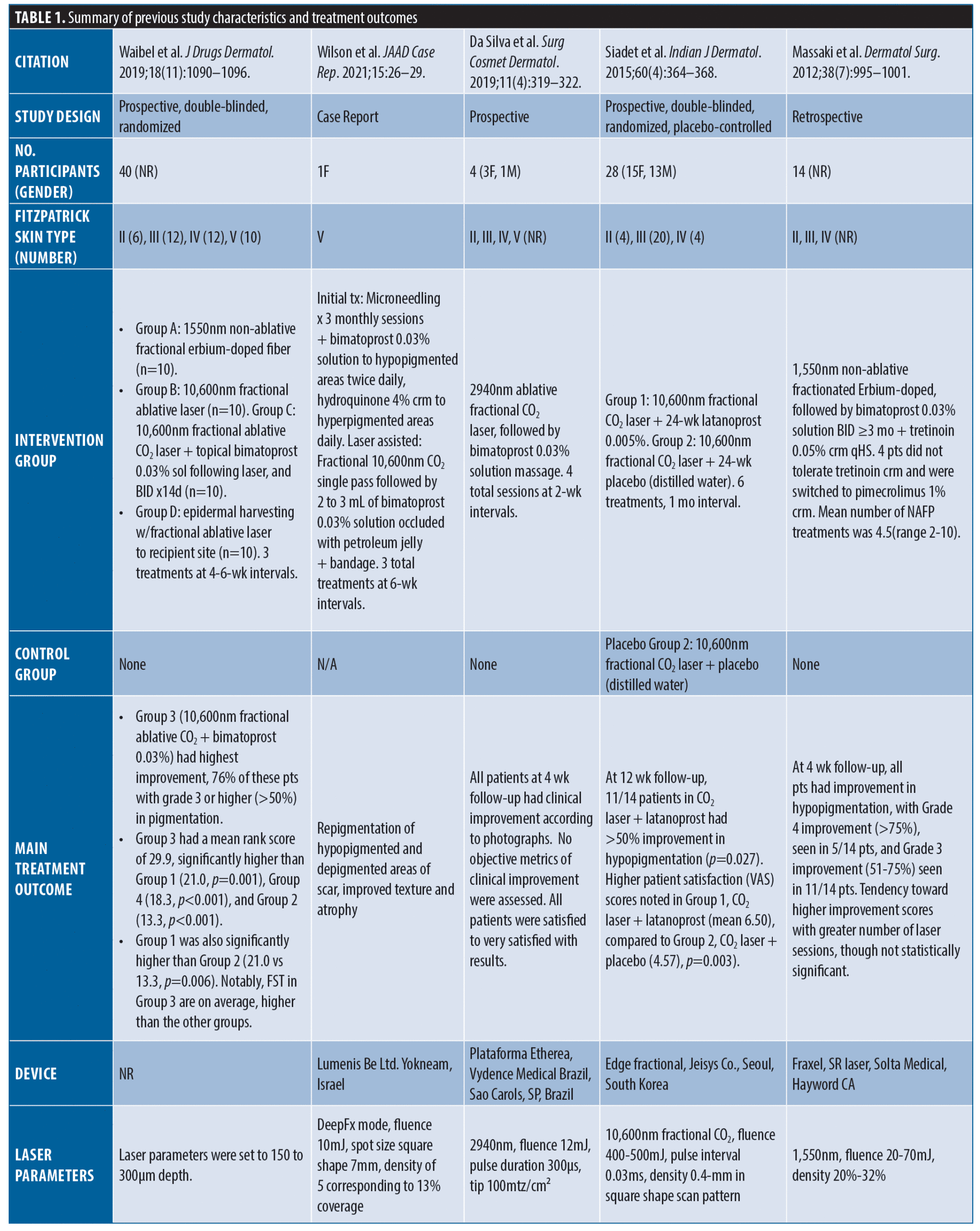
Results. In total, 88 unique studies were reviewed for eligibility. Five studies met inclusion criteria including two prospective, double-blinded, randomized (only one was placebo-controlled), one prospective case series, one retrospective chart review, and one case report; comprising a total of 87 patients. All five studies utilized topical prostaglandin analogs as an adjunctive treatment via laser-assisted delivery. While both, the placebo-controlled and non-placebo-controlled, trials reported more than 75 percent of patients experienced at least 50 percent or more (Grade 3 or higher) improvement, the retrospective study reported 100 percent of patients experienced at least 75 percent or more (Grade 4 or higher) improvement, measured as scar repigmentation. The prospective case series and the reported single case showed overall qualitative improvement in all patients measured as repigmentation of hypopigmented and depigmented scars.
Limitations: Different laser devices, parameters, treatment frequency, and follow-up timepoints.
Conclusion. All studies evaluated demonstrated favorable treatment outcomes with no reported adverse events. Additional, large randomized controlled trials are needed to fully assess the effectiveness and long-term safety of PGF2α agonists for hypopigmented scars.
Keywords. hypopigmented scars; prostaglandin analogs; skin of color; lasers; hyperpigmentation; systematic review; bimatoprost
Hypopigmented scarring can be challenging to treat, largely due to a lack of current efficacious treatments and often transient results.1 Notably, prostaglandin F2α agonists (PGF2α) are FDA-approved as topical ophthalmic solutions for treatment of open-angle glaucoma. More recently, they have been used for desired cosmetic side effects like hypertrichosis and have been shown to induce cutaneous side effects including hyperpigmentation. This effect of PGF2α analog-induced hyperpigmentation has previously provided efficacy in treating vitiligo, and may also provide therapeutic benefit in management of hypopigmented scars.2,3 Thus, we aimed to systematically review the literature to assess the use of prostaglandin analogs in the treatment of hypopigmented scars.
Methods
PubMed/Medline was queried through 10/01/2022 with the following search terms: (bimatoprost AND scar), (latanoprost AND scar), (travoprost AND scar), (prostaglandin analogs AND hypopigmented scars), (PGF2alpha AND hyperpigmentation), (prostaglandin analogs AND hyperpigmentation). Studies met inclusion criteria if they used prostaglandin analogs specifically for the treatment of hypopigmented scars and reported on scar cosmesis. Clinical trials, original interventional studies, case series, and case reports were included. Citations of studies that met inclusion criteria were reviewed to identify additional studies not captured by the database search. Publication duplicates, abstracts, review articles, animal studies, and human studies that did not use PGF2α agonists for the treatment of hypopigmented scars, specifically, were excluded.
Results
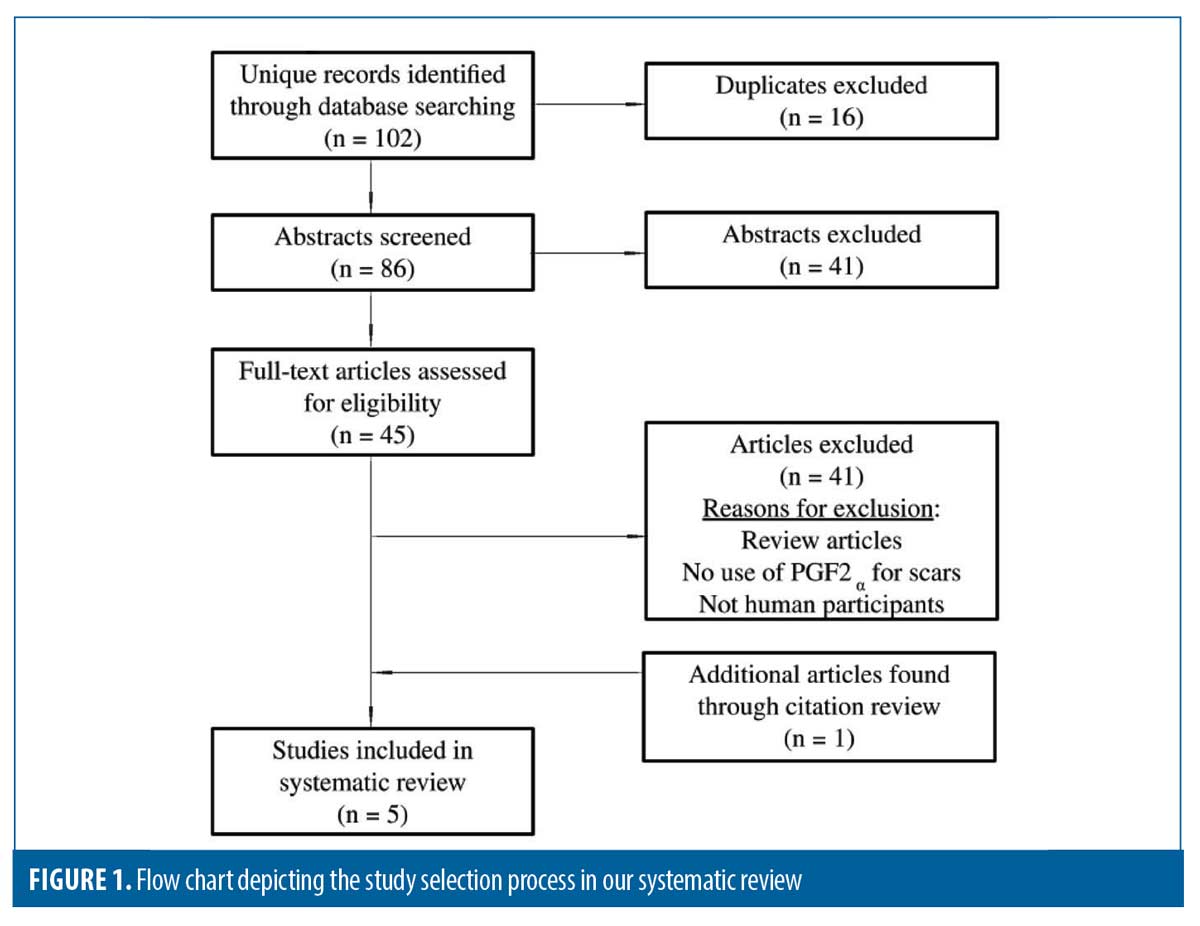
In total, 88 unique studies were reviewed for study eligibility (Figure 1). Five studies met inclusion criteria including two prospective, double-blinded, randomized (one was placebo-controlled), one prospective case series, one retrospective chart review, and one case report; comprising a total of 87 patients (Table 1). Data extracted included patient demographics, Fitzpatrick Skin Type (FST), PGF2α analog used, adjunctive treatments, and patient outcomes.
Siadat et al4 evaluated 28 FST II to IV patients with hypopigmented scars who were treated with six sessions of 10,600nm fractional ablative CO2 laser (FAL) + application of latanoprost 0.005% for 24 weeks versus 10,600nm fractional CO2 laser + distilled water placebo. Eleven of 14 patients demonstrated more than 50 percent improvement in hypopigmentation (p=0.027), correlating with higher patient satisfaction scores compared to CO2 laser + placebo, (p=0.003). Notably, the FAL + bimatoprost group on average had a higher FST compared to other groups, suggesting darker phototypes may respond better.4 Waibel et al. also evaluated treatment of hypopigmented scars in 40 subjects receiving 1,550nm non-ablative fractional erbium-doped laser (NAFL) (n=10), 10,600nm FAL-CO2 (n=10), FAL-CO2 + bimatoprost 0.03% solution, and epidermal harvesting + FAL-CO2. Interestingly, the group treated with FAL-CO2 + bimatoprost had the highest improvement compared to all groups, with 76 percent of these patients exhibiting more than 50 percent improvement in pigmentation (p<0.001).5 Massaki et al,6 similarly demonstrated 50 to 75 percent improvement in the hypopigmented scars of 14 patients treated with a mean of 4.5, 1,550nm NAFL-Erbium treatments, followed by bimatoprost 0.03% solution twice daily for three or more months. Da Silva et al7 treated four patients with four sessions of 2,940nm FAL-CO2 followed by bimatoprost 0.03% solution massage, and noted clinical improvement of hypopigmentation, affirmed by patient satisfaction four weeks posttreatment. Most recently, Wilson et al1 reported a patient with FST V, who developed COVID-19 prone-positioning-induced facial atrophy and hypopigmentation. The patient experienced marked repigmentation of scars five months after initiating bimatoprost 0.03% solution delivered with FAL-CO2 and microneedling.
Discussion
PGF2α agonists are FDA-approved as topical ophthalmic solutions for treating open-angle glaucoma and ocular hypertension, including bimatoprost, latanoprost, and tafluprost, travoprost, and unoprostone.8 Their proposed mechanism of action is the induction of metalloproteinase activity in the ciliary body, which increases aqueous humor outflow and decreases intraocular pressure.9 All five ophthalmic prostaglandin F2α agonists have label warnings for iris and periorbital skin hyperpigmentation. The prostaglandin agonist-induced eyelash changes include increase in length and thickness and has propelled research in dermatology as a potential therapy for alopecia areata of the eyebrows and eyelashes, androgenetic alopecia of the scalp, and chemotherapy-induced eyelash hypotrichosis.10–12 While the efficacy of latanoprost as a hair regrowth therapy is still controversial, bimatoprost (Latisse, Allergan Inc.) has been FDA-approved as 0.03% solution for eyelash hypotrichosis since 2010.13–15 Carboprost, on the other hand, is an FDA-approved PGF2α agonist for the treatment of postpartum hemorrhage administered intramuscularly.16 Since no cases of carboprost-induced hyperpigmentation of skin, hair, or nails have been identified, it may be possible that route of administration plays a role in inducing pigment production and warrants further study. Moreover, studies suggest that excessive periorbital hyperpigmentation induced by PGF2α agonist applied to treat eyelash hypotrichosis is reversible upon treatment discontinuation.17–20
Overall, we have presented recent studies analyzing the efficacy and safety of PGF2α agonists in treating hypopigmented scars, especially by way of laser-assisted delivery, and found that there is substantial preliminary evidence to support their use (Table 1). Although the exact mechanism of action remains unknown, a study by Kapur et al21 analyzing the histopathological findings of bimatoprost-induced periocular skin hyperpigmentation found that increased melanogenesis and increased melanosome transfer to basal keratinocytes, but not melanocyte proliferation or atypia, are the potential underlying mechanisms.21 Similarly, human keratinocytes have been shown to secrete PGE2 and PGF2α in-vitro and stimulate increased melanocyte dendricity via paracrine receptors.22
Studies in mice treated with PGF2α analogs, latanoprost, and isopropyl unoprostone, demonstrated hair follicle conversion from the telogen to the anagen phase 12 days after treatment initiation with cutaneous darkening and pigmentation. Interestingly, each PGF2α showed a distinct pigmentation pattern in all treated mice, but not controls, while treatment with PGE2 agonist demonstrated increased pigmentation in only two out of five mice.23 Similarly, a study in guinea pigs comparing NB-UVB alone, PGF2α analogs alone, and the combination of both found the highest rate of cutaneous pigmentation in combination therapy. All three PGF2α analogs studied (latanoprost, bimatoprost, travoprost) showed equivalent results clinically and histologically.24 Most recently, trans-epidermal delivery of PGF2α agonists has been facilitated by laser adjuncts such as FAL and NAFL resurfacing, further enhancing their role as an adjunctive agent to treat hypopigmented scars in humans.4,5 Taken together, these studies highlight the potential of prostaglandin agonist-induced skin pigmentation and advocate for studies in humans.
Studies in patients with vitiligo place prostaglandin-analogs as a promising therapeutic option for patients with disorders of skin pigmentation. A randomized, double-blind clinical trial with over 31 patients showed significant repigmentation of eyelid vitiligo patches with topical latanoprost gel, but not placebo, twice daily for 12 weeks.25 In addition, a randomized, double-blind, controlled clinical trial with 32 patients showed that bimatoprost 0.03% solution alone, or in combination with mometasone, provided greater repigmentation of nonfacial vitiligo than treatment with mometasone alone.26 Even studies with smaller samples (n=8) have demonstrated at least 50 percent of patients achieve “excellent repigmentation,” and another 25 percent experience “partial repigmentation.”27 Furthermore, a prospective right-left comparative study of 25 patients with vitiligo vulgaris showed more than 50 percent repigmentation was achieved in 13 (52%) patients with combination NB-UVB and topical bimatoprost 0.03% eyedrops versus 10 (40%) patients with NB-UVB alone.28 Although these results seem promising, further molecular and clinical studies are needed to elucidate the exact mechanisms by which immune responses affect skin pigmentation and the long-term safety of these therapies, respectively.3
In conclusion, further studies are needed to elucidate the mechanistic pathways, tissue specificity, and patient demographics that may impact topical prostaglandin analog therapy. Additional, large randomized controlled trials are needed to further evaluate the efficacy and long-term utility of PGF2α agonists in treatment of hypopigmented scars.
References
- Wilson BN, Aleisa A, Menzer C, et al. Bimatoprost drug delivery with fractional laser and microneedling for the management of COVID-19 prone positioning–induced facial atrophy and hypopigmentation. JAAD Case Reports. 2021;15:26–29.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: Beyond the eyelashes. Journal of the American Academy of Dermatology. 2015/04/01/ 2015;72(4):712–716.
- Kanokrungsee S, Khunkhet S, Rojhirunsakool S, et al. Triple combination therapy of narrowband ultraviolet B, fractional carbon dioxide laser and topical bimatoprost 0.01% for non-segmental vitiligo on non-facial areas: A randomized half-body, double-blind, placebo-controlled, comparative study. Dermatol Ther. Jan 2022;35(1):e15198.
- Siadat AH, Rezaei R, Asilian A, et al. Repigmentation of Hypopigmented Scars Using Combination of Fractionated Carbon Dioxide Laser with Topical Latanoprost Vs. Fractionated Carbon Dioxide Laser Alone. Indian J Dermatol. Jul-Aug 2015;60(4):364–368.
- Waibel JS, Rudnick A, Arheart KL, et al. Repigmentation of Hypopigmentation: Fractional Lasers vs Laser-Assisted Delivery of Bimatoprost vs Epidermal Melanocyte Harvesting System. J Drugs Dermatol. Nov 1 2019;18(11):1090–1096.
- Massaki AB, Fabi SG, Fitzpatrick R. Repigmentation of hypopigmented scars using an erbium-doped 1,550-nm fractionated laser and topical bimatoprost. Dermatol Surg. Jul 2012;38(7 Pt 1):995–1001.
- Silva M, Filippo A, Gusmão P. Tratamento de cicatrizes hipocrômicas com laser fracionado ablativo e drug delivery de bimatoprosta: estudo-piloto. Surgical & Cosmetic Dermatology.
- Cai Z, Cao M, Liu K, et al. Analysis of the Responsiveness of Latanoprost, Travoprost, Bimatoprost, and Tafluprost in the Treatment of OAG/OHT Patients. J Ophthalmol. 2021;2021:5586719.
- Winkler NS, Fautsch MP. Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocul Pharmacol Ther. Mar-Apr 2014;30(2-3):102–109.
- Barron-Hernandez YL, Tosti A. Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia. Expert Opin Investig Drugs. Apr 2017;26(4):515–522.
- Vila TO, Camacho Martinez FM. Bimatoprost in the treatment of eyelash universalis alopecia areata. Int J Trichology. Jul 2010;2(2):86–88.
- Zaher H, Gawdat HI, Hegazy RA, et al. Bimatoprost versus Mometasone Furoate in the Treatment of Scalp Alopecia Areata: A Pilot Study. Dermatology. 2015;230(4):308–313.
- Glaser DA, Hossain P, Perkins W, et al. Long-term safety and efficacy of bimatoprost solution 0.03% application to the eyelid margin for the treatment of idiopathic and chemotherapy-induced eyelash hypotrichosis: a randomized controlled trial. Br J Dermatol. 2015;172(5):1384–1394.
- Goodarzi A, Balasi J, Mashayekhi F, et al. Latanoprost in treatment of alopecia areata and androgenic alopecia: A comprehensive review. Pakistan Journal of Medical and Health Sciences. 04/04 2021;17:1535.
- Law SK. Bimatoprost in the treatment of eyelash hypotrichosis. Clin Ophthalmol. Apr 26 2010;4:349–358.
- Chen Y, Jiang W, Zhao Y, et al. Prostaglandins for Postpartum Hemorrhage: Pharmacology, Application, and Current Opinion. Pharmacology. 2021;106(9-10):477–487.
- Chou SY, Chou CK, Kuang TM, et al. Incidence and severity of iris pigmentation on latanoprost-treated glaucoma eyes. Eye (Lond). Jul 2005;19(7):784–787.
- Wand M, Ritch R, Isbey EK, et al. Latanoprost and Periocular Skin Color Changes. Archives of Ophthalmology. 2001;119(4):614–615.
- Doshi M, Edward DP, Osmanovic S. Clinical course of bimatoprost-induced periocular skin changes in Caucasians. Ophthalmology. Nov 2006;113(11):1961–1967.
- Inoue K, Shiokawa M, Higa R, et al. Adverse periocular reactions to five types of prostaglandin analogs. Eye (Lond). Nov 2012;26(11):1465–1472.
- Kapur R, Osmanovic S, Toyran S, et al. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. Nov 2005;123(11):1541–1546.
- Scott G, Leopardi S, Printup S, et al. Proteinase-Activated Receptor-2 Stimulates Prostaglandin Production in Keratinocytes: Analysis of Prostaglandin Receptors on Human Melanocytes and Effects of PGE<sub>2</sub> and PGF<sub>2α</sub> on Melanocyte Dendricity. Journal of Investigative Dermatology. 2004;122(5):1214–1224.
- Sasaki S, Hozumi Y, Kondo S. Influence of prostaglandin F2α and its analogues on hair regrowth and follicular melanogenesis in a murine model. Experimental Dermatology. 2005;14(5):323–328.
- Anbar T, El-Ammawi T, Barakat M, et al. Skin pigmentation after NB-UVB and three analogues of prostaglandin F2α in guinea pigs: a comparative study. Journal of the European Academy of Dermatology and Venereology. 2010;24(1):28–31.
- Nowroozpoor Dailami K, Hosseini A, Rahmatpour Rokni G, et al. Efficacy of topical latanoprost in the treatment of eyelid vitiligo: A randomized, double-blind clinical trial study. Dermatol Ther. Jan 2020;33(1):e13175.
- Grimes PE. Bimatoprost 0.03% Solution for the Treatment of Nonfacial Vitiligo. J Drugs Dermatol. Jun 1 2016;15(6):703–710.
- Jha AK, Prasad S, Sinha R. Bimatoprost ophthalmic solution in facial vitiligo. J Cosmet Dermatol. Jun 2018;17(3):437–440.
- Sharma S, Parsad D, Bhattacharjee R, et al. A prospective right-left comparative study to evaluate the efficacy and tolerability of combination of NB-UVB and topical bimatoprost 0.03% eye drops versus NB-UVB given alone in patients of vitiligo vulgaris. J Eur Acad Dermatol Venereol. Aug 2018;32(8):e330-e331.