 J Clin Aesthet Dermatol. 2022;15(6 Suppl 1):S19–S25,31
J Clin Aesthet Dermatol. 2022;15(6 Suppl 1):S19–S25,31
by Amylee Martin, BS; Akshitha Thatiparthi, BS; Jeffrey Liu, BS; and Jashin J. Wu, MD
FUNDING: No funding was provided for the preparation of this article.
DISCLOSURES: Mses. Martin and Thatiparthi and Mr. Liu report no conflicts of interest relevant to the content of this article. Dr. Wu is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant, Dr. Reddy’s Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, Valeant Pharmaceuticals North America LLC, and Zerigo Health.
ABSTRACT: Objective. Although biologics are highly effective in the treatment of psoriasis, some patients consistently fail monotherapy. For these patients, combination therapy is commonly employed. However, evidence-based recommendations for combination therapy in the treatment of psoriasis with interleukin-17 (IL-17) inhibitors are currently lacking. Therefore, we aimed to conduct a systematic review of existing literature discussing the efficacy and safety of IL-17 inhibitors in combination with other therapeutic modalities in the treatment of psoriasis.
Methods. By way of a search of PubMed, Cochrane, and Web of Science databases in March 2021, we identified peer-reviewed articles with data on the safety and/or efficacy of IL-17 inhibitor combination therapies in adults with psoriasis and/or psoriatic arthritis (PsA). A modified version of the 2011 Oxford Centre for Evidence-Based Medicine Scheme was utilized for assessing study quality.
Results. Twenty-four articles with a total of 3,154 patients met inclusion/exclusion criteria. These articles comprised six post-hoc/subgroup analyses of randomized controlled trials (RCTs), four uncontrolled clinical trials, three case series, and 11 case reports that provided data on IL-17 inhibitor therapy in combination with conventional disease-modifying antirheumatic drugs (cDMARDs), apremilast, acitretin, topical therapy, phototherapy, and/or medications for comorbid diseases.
Limitations. Our results are limited by the lack of data from RCTs.
Conclusion. Although cDMARDs are often used in psoriasis combination therapies, the current literature suggests concomitant cDMARDs with IL-17 inhibitor therapy provides no added benefit compared to IL-17 inhibitor monotherapy. However, IL-17 inhibitor in combination with apremilast or acitretin shows efficacy and safety in case series/reports and may allow for a reduction in medication dosing and/or frequency, thereby minimizing costs and adverse events. Future RCTs investigating IL-17 inhibitor therapy in combination with acitretin or apremilast are warranted.
Keywords. Interleukin-17 inhibitor, IL-17 inhibitor, psoriasis, combination therapy, psoriatic arthritis
Psoriasis, a chronic inflammatory skin disease associated with a multitude of comorbidities including psoriatic arthritis (PsA), affects 2 to 3 percent of the United States (US) population.1,2 Several effective biologic therapies have emerged for psoriasis within the last decade.3 However, some individuals with psoriasis fail or stop responding to some or all available biologics, thereby warranting combination therapy.4,5 In patients with rheumatoid arthritis, administration of methotrexate (MTX) at low doses in combination with biologics reduces the rate of antidrug antibody formation.3 In addition to increased efficacy, combination therapy may also reduce drug dosage and administration frequency, leading to lower cost and risk of toxicity.4,5
Though there have been a limited number of trials investigating the efficacy and safety of combination therapies in psoriasis, this treatment strategy is commonly used in clinical practice.4,6 One study noted combination therapy use in up to 42.9 percent of patients with moderate-to-severe plaque psoriasis.7 Moreover, individuals utilizing Medicaid and identifying as Black are more likely to be on combination therapy, as are individuals with PsA.6
Trials investigating the combination therapy using MTX and tumor necrosis factor-alpha (TNF-α) inhibitors in psoriasis have shown benefits.8,9 However, combination therapy with interleukin-17 inhibitors (IL-17i), a newer class of biologics, is less explored. Currently, there are three US Food and Drug Administration (FDA)-approved IL-17i (secukinumab, brodalumab, ixekizumab) for the treatment of moderate-to-severe psoriasis.10 Since IL-17i and combination therapy are both frequently used in practice, guidance is needed on the risks and benefits associated with IL-17i combination therapy.7,11 Therefore, the goal of this review is to evaluate and summarize the available data regarding the safety and efficacy of IL-17i combination therapy in adults with psoriasis and/or PsA.
Methods
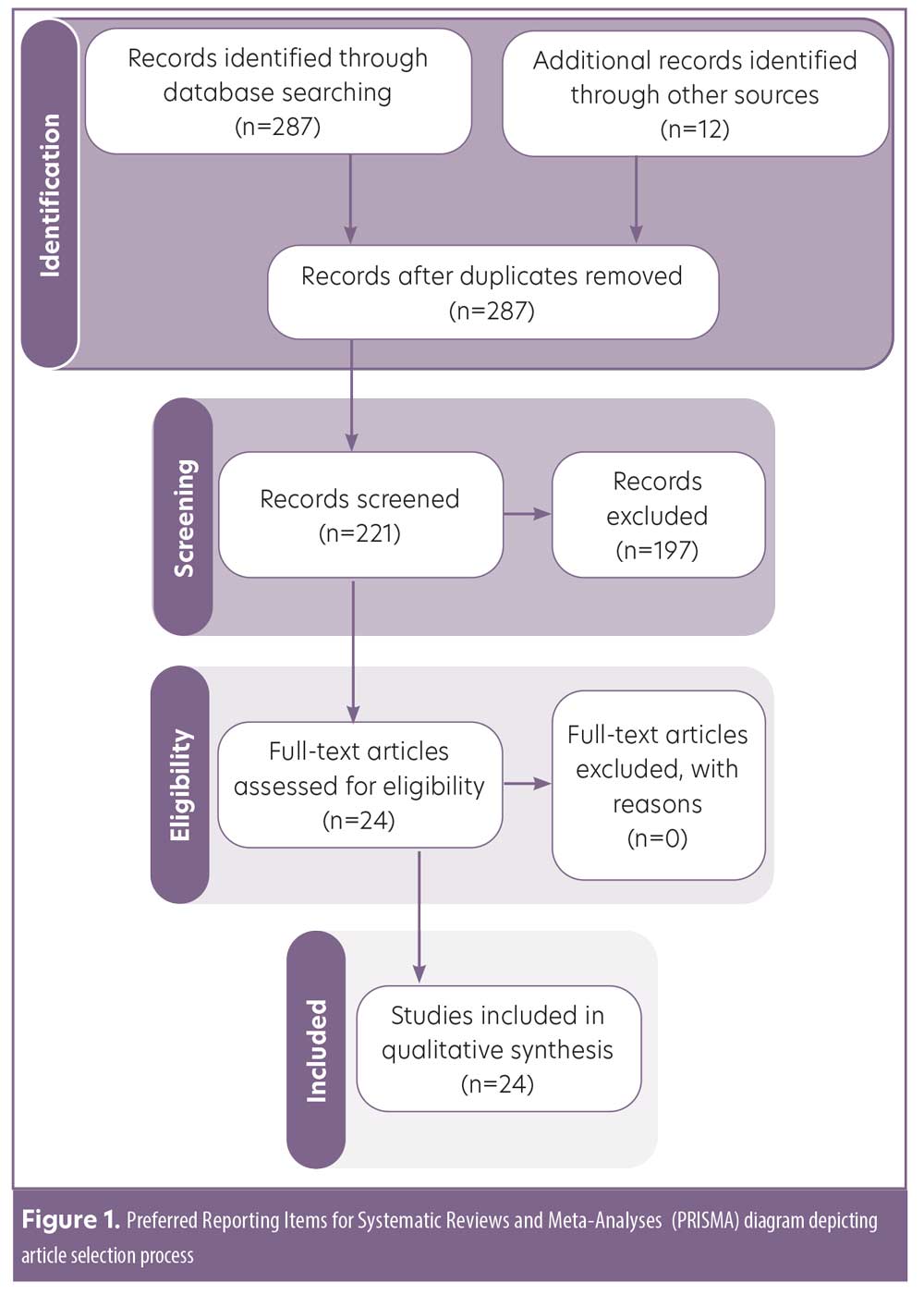
A primary literature search was conducted in March 2021 using PubMed/MEDLINE, Cochrane, and Web of Science bibliometric databases in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 The following search terms were employed: psoriasis AND secukinumab OR ixekizumab OR brodalumab OR interleukin-17 inhibitor AND combined OR combination. All peer-reviewed articles with data on the safety and/or efficacy of IL-17i combination therapy in adults with psoriasis and/or PsA were considered for inclusion (Figure 1). Due to the limited data available on this topic, we included case series and case reports. No date criteria were applied. Articles not in English and/or not in humans were excluded, as were meeting abstracts, commentaries, and reviews. References of included articles were examined for additional studies meeting inclusion/exclusion criteria. All articles were screened and evaluated by A.M. with oversight from J.J.W. Data on patient characteristics, treatment regimen, treatment efficacy, and adverse events were extracted. Lastly, level of evidence (LOE) was assessed using a modified version of the 2011 Oxford Centre for Evidence-Based Medicine Scheme:13 1 (highest)=systematic review of randomized trials; 2=randomized trial or observational study with dramatic effect; 3=nonrandomized controlled cohort/follow up study; 4=case series, case-control, or historically controlled studies; 5 (lowest)=case report.
Results
Overall results. Twenty-four articles meeting inclusion/exclusion criteria, with a total of 3,154 patients, were included in this review. These articles consisted of six post-hoc/subgroup analyses of randomized clinical trials (RCTs), four uncontrolled clinical trials, three case series, and 11 case reports (Table 1). Forty-six percent of the articles were rated with an LOE of 5, 25 percent with an LOE of 2, 17 percent with an LOE of 3, and 12 percent with an LOE of 4. In these articles, the following medications were combined with IL-17i: apremilast (n=7), acitretin (n=3), medication for comorbidity (n=3), topical treatments (n=2), or phototherapy (n=1). Additionally, the six included RCTs examined IL-17i combination therapy with conventional disease-modifying antirheumatic drugs (cDMARDs)/MTX in PsA subjects (Table 1).
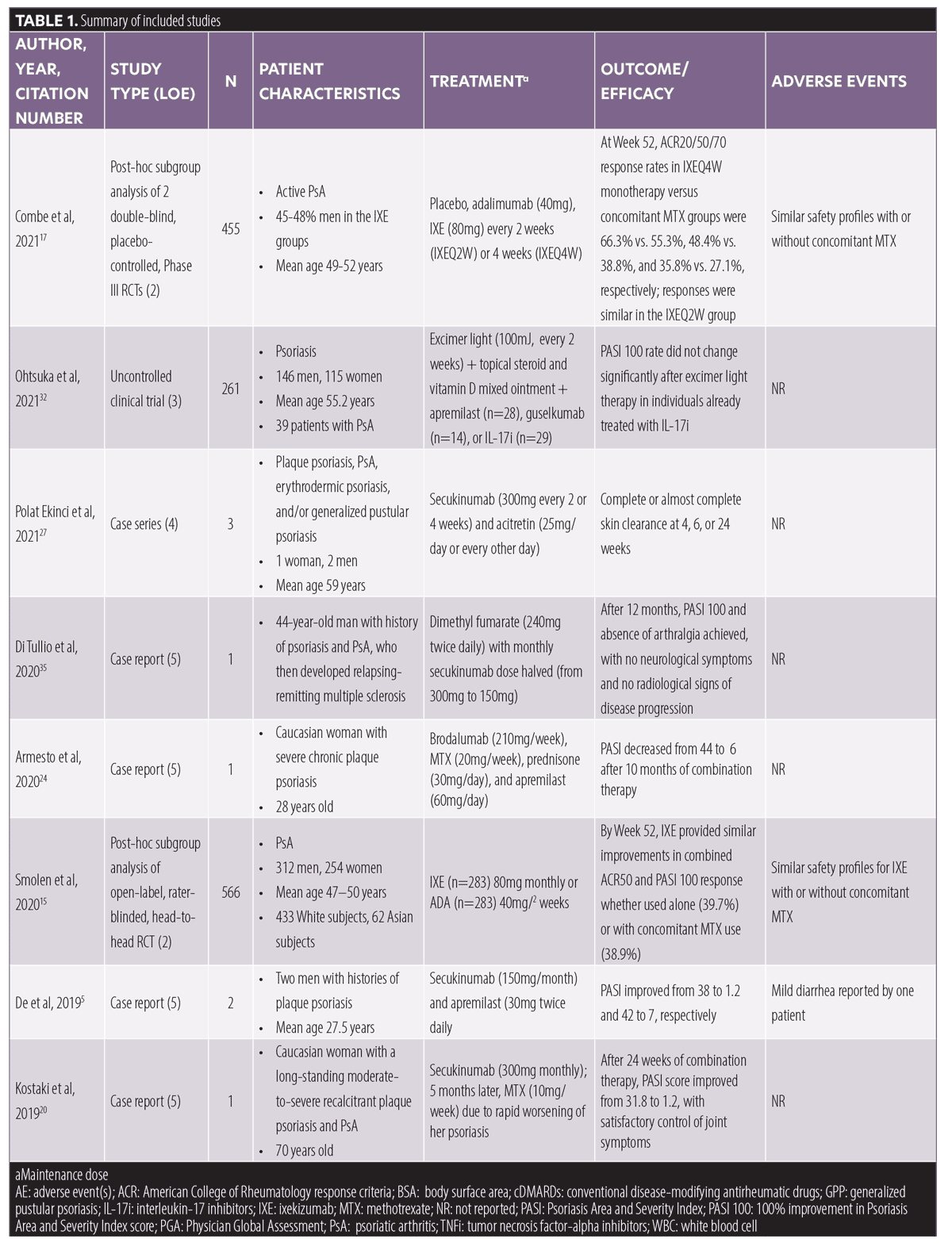
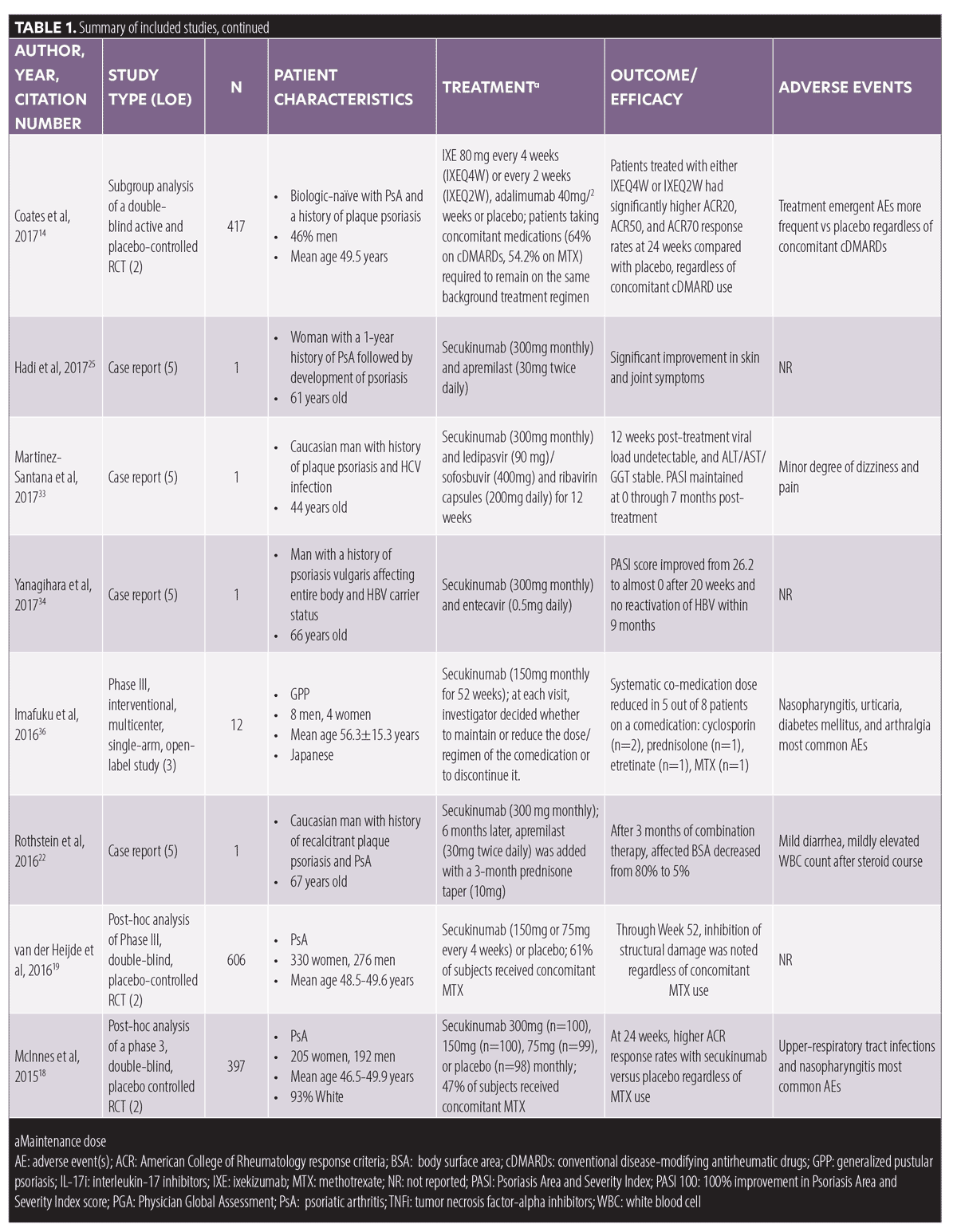
Combination therapy with cDMARDs/MTX. Data on the efficacy and safety of ixekizumab in combination with cDMARDs, predominately MTX, were extracted from four RCTs with an aggregate of 1,801 patients with PsA.14–17 The largest of these studies (n=566) was a post-hoc subgroup analysis of a rater-blinded, head to head RCT comparing ixekizumab and adalimumab in biologic-naïve patients with PsA (SPIRIT-H2H).14 This study reported similar combined 50-percent or greater improvement in American College of Rheumatology scale score (ACR-50) and 100-percent improvement in Psoriasis Area and Severity Index (PASI-100) score responses (38.9% without MTX vs. 39.7% with MTX) for ixekizumab at 52 weeks, with a comparable side effect profile, regardless of concomitant MTX use.14 In a subgroup analysis of a double-blind, active (adalimumab) and placebo-controlled RCT, researchers investigated the efficacy of ixekizumab administered every two (IXEQ2W) or four weeks (IXEQ4W) in 417 biologic-naïve subjects with PsA (SPIRIT-P1).15 This study reported significantly higher ACR-20, ACR-50, and ACR-70 response rates for both IXE20W and IXE40W compared to the placebo, regardless of concomitant cDMARD use. Similarly, a subgroup analysis of SPIRIT-P2 trial data (double-blind, placebo-controlled, Phase III RCT of 363 subjects with PsA who responded inadequately or were intolerant of TNFi) demonstrated IXEQ2W and IXEQ4W were effective, with consistent side effects, when used alone or with concomitant cDMARDs.16 Lastly, a post-hoc subgroup analysis (n=455) of SPIRIT-P1 and SPIRIT-P2 concluded that ixekizumab demonstrated sustained efficacy at 52 weeks, regardless of concomitant MTX.17 In this study, 55 percent versus 66 percent, 39 percent versus 48 percent, and 27 percent versus 36 of percent subjects in the IXE4QW group achieved ACR-20, ACR-50, or ACR-70 responses with or without MTX, respectively. Comparable results were obtained for the IXE2QW group, and the ixekizumab safety profile was similar with or without MTX.17
Two RCTs of patients with PsA (n=1,003) provided data on the efficacy and safety of secukinumab and MTX combination therapy.18,19 A post-hoc analysis of a Phase III RCT of 397 subjects with PsA (FUTURE 2) reported higher ACR responses with secukinumab (300mg, 150mg, or 75mg) compared to the placebo at 24 weeks, with or without MTX.18 Similarly, an exploratory post-hoc analysis of a Phase III RCT of 606 patients with PsA demonstrated secukinumab (150mg or 75mg) inhibits structural damage regardless of concomitant MTX use.19
One case series and one case report described secukinumab and MTX combination therapy in a total of three patients with psoriasis and comorbid PsA.20,21 Notably, one patient demonstrated a significant improvement of skin and joint symptoms after MTX was added to secukinumab subsequent to a relapse on secukinumab monotherapy,20 and one patient experienced worsening of PsA when MTX (15mg) was tapered after six weeks of treatment with secukinumab.21
Combination therapy with apremilast. Two case series and five case reports treated an aggregate of 11 patients with psoriasis with IL-17i and apremilast combination therapy.5,21–26 Of these, six case series/reports found apremilast and IL-17i combination therapy to be effective for treating recalcitrant psoriasis and/or PsA,5,22–26 while one case series noted inadequate response to this combination therapy in one patient.21 The majority of studies combined secukinumab with apremilast;5,22,23,25,26 however, one case report combined apremilast with brodalumab with favorable results.24 Apremilast and IL-17i combination therapy was well tolerated, with mild diarrhea being the most commonly reported adverse event.5,21–26
Combination therapy with acitretin. Acitretin (25mg/day or every other day or 10mg/day) and secukinumab (300mg/2 for 4 weeks) combination therapy was successfully used to treat five patients with plaque, erythrodermic, generalized pustular, and/or annular pustular psoriasis.27–29 Interestingly, low-dose acitretin (25mg/day) and secukinumab (300mg monthly) combination therapy was initiated in one patient with generalized pustular psoriasis and hepatosteatosis who experienced elevation of liver enzymes on monotherapy with acitretin 50mg/day.27 Acitretin and secukinumab combination therapy was well tolerated with no reported adverse events.27–29
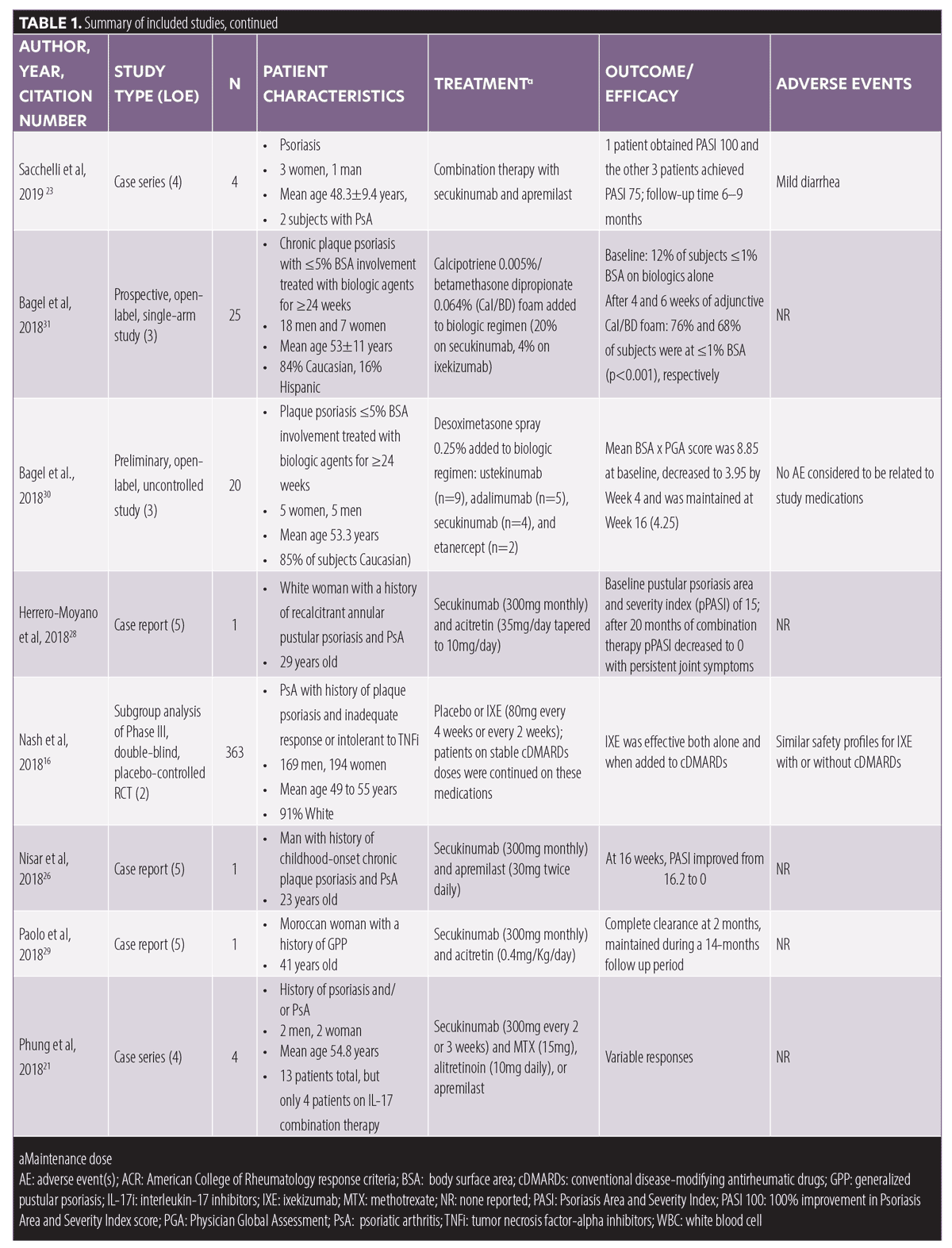
Combination therapy with topicals and phototherapy. Two uncontrolled clinical trials investigated the benefits of topical therapy in patients being treated with biologics in an aggregate of 45 patients, with 10 of these patients being on IL-17i.30,31 In one of these studies, which included 20 patients with mild plaque psoriasis, four patients who had been treated with secukinumab for 24 weeks or longer achieved a decrease in the mean of the product of BSA and Physician Global Assessment (PGA) score from 8.85 to 3.95, after four weeks of desoximetasone spray 0.25% application.30 In the other uncontrolled clinical trial,31 25 patients with mild plaque psoriasis (BSA≤ 5%) who had been treated with a biologic agent (20% secukinumab, 4% ixekizumab) for 24 weeks or longer without adequate response were enrolled in a 16-week study in which they were administered calcipotriene 0.005%/betamethasone dipropionate 0.064% (Cal/BD) foam in addition to their biologic. At Week 16, 68 percent of the patients achieved a total BSA of one percent or less, compared to 12 percent at baseline (p<0.001).31
Finally, in an uncontrolled trial, 261 patients with psoriasis were treated with a mixed ointment of topical steroid and vitamin D. Researchers observed no significant change in the PASI-100 rate in patients on an IL-17i when adding excimer light therapy to the treatment regimen compared to IL-17i monotherapy (13 of 29 [44.8%] no light therapy versus 14 of 29 [48.3%] light therapy).32
Combination therapy with drugs prescribed for comorbidities. We encountered two case reports that demonstrated favorable results when combining secukinumab (300mg monthly) with antiviral treatment in patients with psoriasis and concomitant hepatitis B and C viral (HBV and HCV) infection.33,34 The first case presented a 44-year-old man with a history of plaque psoriasis and HCV, who was treated with secukinumab (300mg monthly) in combination with ledipasvir (90mg)/sofosbuvir (400mg) and ribavirin capsules (200mg) daily for 12 weeks.33 The viral load remained undetectable and liver enzyme remained stable at 12 weeks post-treatment, with a PASI of zero at seven months; however, the patient reported minor dizziness and pain.33 The other case report presented a 66-year-old man with severe psoriasis, who was also a carrier for HBV.34 The patient achieved a decrease in PASI from 26.2 to near zero after 20 weeks of secukinumab (300mg monthly) and entecavir (0.5mg daily), with no adverse events and no HBV reactivation occurring within a nine-month period.34
Additionally, in a case report of a 44-year-old man with psoriasis, PsA, and multiple sclerosis (MS), the authors reported positive outcomes when combining secukinumab (300mg monthly) and dimethyl fumarate (240 twice daily).35 After two years of successful treatment with secukinumab, the patient was diagnosed with relapsing-remitting MS, which prompted the addition of dimethyl fumarate and reduction of secukinumab dose to 150mg/month.35 Twelve months later, PASI-100 was maintained with no arthralgias, neurological symptoms, or adverse events. Notably, dimethyl fumarate is only approved for the treatment of psoriasis in Germany and is undergoing clinical trials in the United States.
Other studies. One Phase III, interventional, multicenter, single-arm study administered secukinumab (150mg monthly) for 52 weeks to 12 patients with generalized pustular psoriasis.36 Out of the eight patients who were taking a comedication, a reduction in the comedication dose was observed in five patients: cyclosporin (n=2), prednisolone (n=1), etretinate (n=1) and MTX (n=1).36
Discussion
Although combination therapy with cDMARDs is commonly used for managing patients with psoriasis and/or PsA in clinical practice, analysis of study results revealed continuing cDMARDs when initiating IL-17i therapy provides no added benefit compared to IL-17i monotherapy.14-19 However, the adverse event profile for IL-17i, with or without cDMARDs, was comparable, suggesting this drug combination therapy is safe.14–19 Interestingly, secukinumab has been reported to have low immunogenicity, which may explain why IL-17i therapy is effective regardless of cDMARD use.37,38 It should be noted the majority of studies on this topic provided data from post-hoc and subgroup analyses of trials, which included subjects receiving cDMARDs prior to enrollment without adequate response.14–19 Therefore, future studies that are designed to investigate the efficacy of cDMARDs and IL-17i combination therapy in patients with psoriasis who are naïve to cDMARDs are needed to confirm the findings from our review.
Additionally, evidence from case series and case reports suggests secukinumab and apremilast combination therapy is a desirable treatment option for patients with plaque psoriasis and/or PsA who have failed several monotherapies.5,21–26 Notably, combining apremilast with secukinumab allows for a reduction in the dose of secukinumab, minimizing costs and side effects (Table 2).5 Moreover, preliminary reports of patients with recalcitrant plaque psoriasis, erythrodermic psoriasis, pustular psoriasis, and/or PsA demonstrated low-dose acitretin and secukinumab combination therapy may be beneficial for patients who respond well to acitretin but develop adverse events at higher doses.27–29 Furthermore, topical therapies should be considered in patients with mildly persistent psoriatic lesions despite treatment with an IL-17i (Table 2).30,31
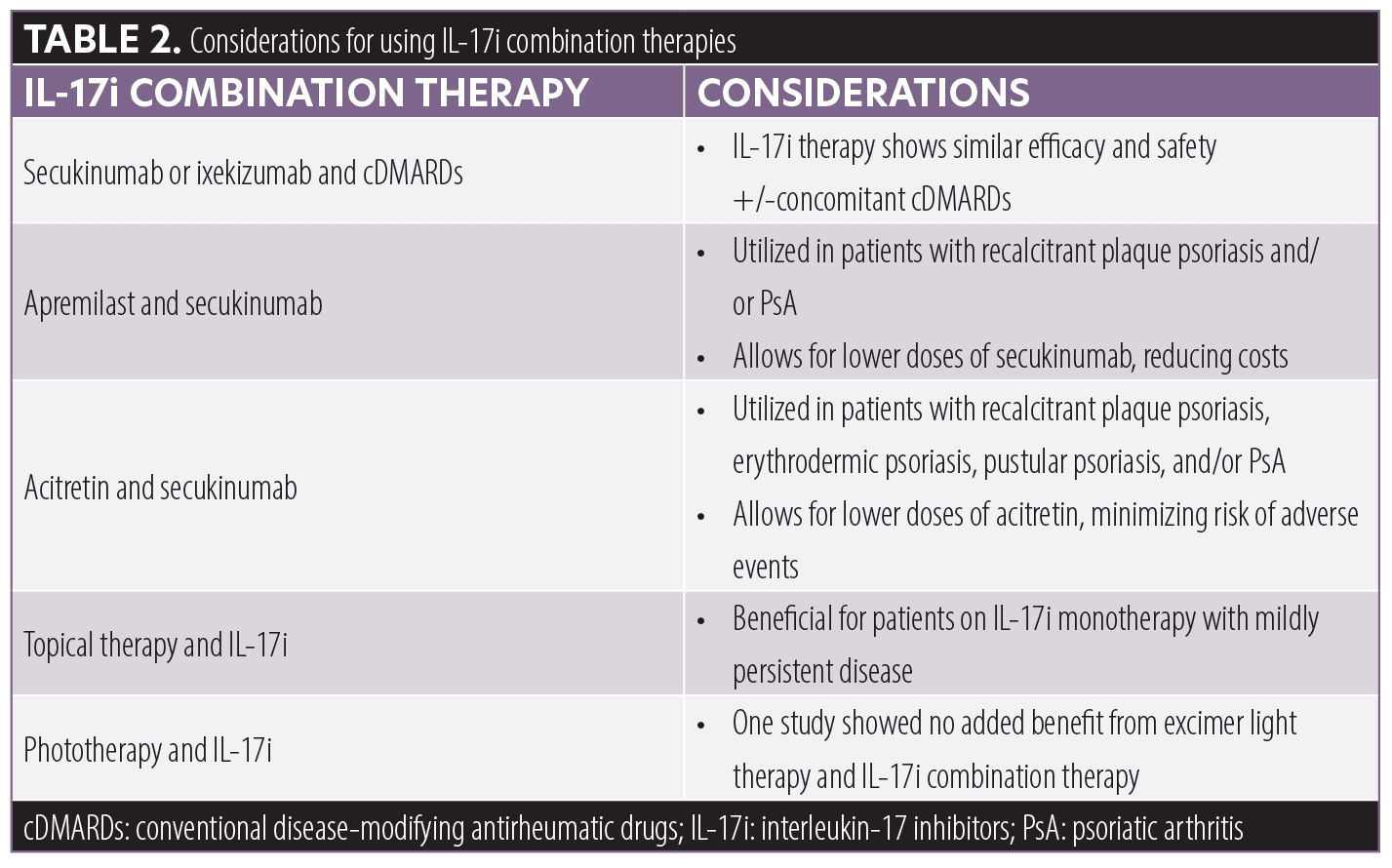
Interestingly, a study investigating Th17 mediated autoimmunity demonstrated increased efficacy from dual inhibition of interleukins-17 and -23, compared to either cytokine alone.39 Moreover, a new biologic that targets both TNF and IL-17A, referred to as ABT-122, is being investigated in Phase I and II trials.40,41 Therefore, combination of different biologic classes may be effective for patients with severe, recalcitrant psoriasis who have failed the available monotherapies.
Limitations. This review is limited by a lack of high-quality studies designed with the intent to investigate efficacy and safety of IL-17i combination therapy. Moreover, the majority of evidence is from case series/case reports, thereby limiting the impact and generalizability of our results. However, our summary and evaluation of the evidence that is currently available on this topic provides insight into a treatment strategy that is commonly utilized in clinical practice.
Conclusion
The current literature demonstrates no added benefit from continuing cDMARDs with IL-17i therapy compared to IL-17i monotherapy in the treatment of PsA and/or psoriasis. However, for select patients with recalcitrant psoriasis, IL-17i combination therapy with apremilast or acitretin shows promising preliminary results in case series/reports and may allow for reduction in medication dosing/frequency. Future studies investigating the efficacy of combination therapy with IL-17i and apremilast or acitretin are needed to expand upon our findings.
References
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the U.S. 2009–2010 and 2013–2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. October 2020. doi:10.1016/j.jaad.2020.10.035
- Feldman SR, Zhang J, Martinez DJ, et al. Real-world treatment patterns and healthcare costs of biologics and apremilast among patients with moderate-to-severe plaque psoriasis by metabolic condition status. J Dermatolog Treat. 2021;32(2):203–211.
- Farhangian ME, Feldman SR. Immunogenicity of biologic treatments for psoriasis: therapeutic consequences and the potential value of concomitant methotrexate. Am J Clin Dermatol. 2015;16(4):
285–294. - Gustafson CJ, Watkins C, Hix E, Feldman SR. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14(1):
9–25. - De A, Das S, Dhoot D, Sarda A. Apremilast coadministered with secukinumab for safe and effective control of psoriasis with resultant reduction of maintenance dose of the biologic. Indian J Dermatol. 2019;64(3):239–241.
- Bonomo L, Abittan BJ, Hashim PW, et al. combination use of systemic therapies in psoriasis: baseline characteristics from the Corrona Psoriasis Registry. J Drugs Dermatol. 2019;18(8):731–740.
- Feldman SR, Zhang J, Martinez DJ, et al. Real-world biologic and apremilast treatment patterns and healthcare costs in moderate-to-severe plaque psoriasis. Dermatol Online J. 2021;27(1):13030/qt03t0s9j6.
- Zachariae C, Mørk NJ, Reunala T, et al. The combination of etanercept and methotrexate increases the effectiveness of treatment in active psoriasis despite inadequate effect of methotrexate therapy. Acta Derm Venereol. 2008;88(5):495-501.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167(3):649–657.
- Thomas LW, Lee EB, Wu JJ. Systematic review of anti-drug antibodies of IL-17 inhibitors for psoriasis. J Dermatolog Treat. 2019;30(2):
110–116. - Lockshin B, Cronin A, Harrison RW, et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: the Corrona Psoriasis Registry. Dermatol Ther. January 2021. doi:10.1111/dth.14808.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6(7): e1000097.
- OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009. Accessed 12 May 2022.
- Smolen JS, Sebba A, Ruderman EM, et al. Efficacy and safety of ixekizumab with or without methotrexate in biologic-naïve patients with psoriatic arthritis: 52-week results from SPIRIT-H2H study. Rheumatol Ther. 2020;7(4):1021-1035.
- Coates LC, Kishimoto M, Gottlieb A, et al. Ixekizumab efficacy and safety with and without concomitant conventional disease-modifying antirheumatic drugs (cDMARDs) in biologic DMARD (bDMARD)-naïve patients with active psoriatic arthritis (PsA): results from SPIRIT-P1. RMD Open. 2017;3(2):e000567. 7
- Nash P, Behrens F, Orbai AM, et al. Ixekizumab is efficacious when used alone or when added to conventional synthetic disease-modifying antirheumatic drugs (cDMARDs) in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor inhibitors. RMD Open. 2018;4(2):692.
- Combe B, Tsai TF, Huffstutter JE, et al. Ixekizumab, with or without concomitant methotrexate, improves signs and symptoms of PsA: Week 52 results from Spirit-P1 and Spirit-P2 studies. Arthritis Res Ther. 2021;23(1).
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, Phase III trial. Lancet. 2015;386(9999):1137-1146.
- van der Heijde D, Landewé RB, Mease PJ, et al. Brief report: secukinumab provides significant and sustained inhibition of joint structural damage in a Phase III study of active psoriatic arthritis. Arthritis Rheumatol. 2016;68(8):
1914–1921. - Kostaki D, Aquila E, Macaluso L, et al. Optimizing secukinumab treatment in psoriasis with concomitant methotrexate administration: minireview and a case report. Case Rep Dermatol. 2019;11(1):17-22.
- Phung M, Georgakopoulos JR, Ighani A, et al. Secukinumab dose optimization in adult psoriasis patients: a retrospective, multicenter case series. JAAD Case Reports. 2018;4(4):310–313.
- Rothstein BE, McQuade B, Greb JE, et al. Apremilast and secukinumab combined therapy in a patient with recalcitrant plaque psoriasis. J Drugs Dermatol. 2016;15(5):648–649.
- Sacchelli L, Patrizi A, Loi C, Bardazzi F. Combination therapy of apremilast and secukinumab in patients with moderate-to-severe, recalcitrant plaque psoriasis. Clin Exp Dermatol. 2019;44(7):e243–e244.
- Armesto S, González Vela C, Sánchez J, et al. Treating multidrug-resistant psoriasis with brodalumab, apremilast, methotrexate and prednisone combination therapy in the COVID-19 pandemic. Dermatol Ther. 2020 Nov;33(6):e14464.
- Hadi A, Lebwohl M. Secukinumab and apremilast combination therapy for recalcitrant psoriasis. J Psoriasis Psoriatic Arthritis. 2017;2(2):59–61.
- Nisar MK. Combining secukinumab and apremilast to successfully treat refractory psoriatic skin and joint disease: a novel approach. Eur J Rheumatol. 2019;6(1):57–58.
- Polat Ekinci A, Bölük KN, Babuna Kobaner G. Secukinumab and acitretin as a combination therapy for three clinical forms of severe psoriasis in multidrug refractory patients: a case series of high efficacy and safety profile. Dermatol Ther. 2021;34(1):e14704.
- Herrero-Moyano M, Capusan TM, Martínez-Mera C, et al. Recalcitrant annular pustular psoriasis associated with psoriatic arthritis successfully treated with secukinumab. JAAD Case Reports. 2018;4(8):842-844.
- Paolo G. Secukinumab associated with acitretin in generalized pustular psoriasis. Arch Clin Case Stud. 2018;1(2).
- Bagel J, Nelson E, Keegan B. Open-label, observational pilot study evaluating desoximetasone topical spray 0.25% twice daily in patients with psoriasis being treated with biologic agents. J Psoriasis Psoriatic Arthritis. 2018;3(1):10–14.
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17(8):
845–850. - Ohtsuka T, Kotani H. Additional effect of excimer light therapy in patients with psoriasis under other therapies. J Dermatol. 2021;00:1346-8138.15802.
- Martinez-Santana V, Rodriguez-Murphy E, Smithson A, et al. Efficacy and safety of direct-acting antiviral agents when combined with secukinumab. Eur J Hosp Pharm. 2018;25(1):53-56.
- Yanagihara S, Sugita K, Yoshida Y, et al. Psoriasis vulgaris in a Hepatitis B virus carrier successfully treated with secukinumab and entecavir combination therapy. Eur J Dermatology. 2017;27(2):185-186.
- Di Tullio F, Odorici G, Lasagni C, et al. Combination treatment with secukinumab and dimethyl fumarate in a patient with psoriasis and recent diagnosis of multiple sclerosis. Dermatol Ther. 2020;33(6):e13943.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from Phase III open-label multicenter Japanese study. J Dermatol. 2016;43(9):1011–1017.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176(3):752–758.
- Spindeldreher S, Maillère B, Correia E, et al. Secukinumab demonstrates significantly lower immunogenicity potential compared to ixekizumab. Dermatol Ther (Heidelb). 2018;8(1):57–68.
- Mangan PR, Su LJ, Jenny V, et al. Dual inhibition of interleukin-23 and interleukin-17 offers superior efficacy in mouse models of autoimmunities. J Pharmacol Exp Ther. 2015;354(2):152–165.
- Khatri A, Othman AA. Population pharmacokinetics of the TNF-α and IL-17A dual-variable domain antibody ABT-122 in healthy volunteers and subjects with psoriatic or rheumatoid arthritis: analysis of Phase I and II clinical trials. J Clin Pharmacol. 2018;58(6):
803–813. - Mease PJ, Genovese MC, Weinblatt ME, et al. Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol. 2018;70(11):1778–1789.

