 J Clin Aesthet Dermatol. 2020;13(12):38–40.
J Clin Aesthet Dermatol. 2020;13(12):38–40.
by María del Mar Meléndez González, BS; Christopher Sayed, MD; and Pushkar Phadke, MD
Ms. Meléndez González is with the Universidad Central del Caribe School of Medicine in Bayamon, Puerto Rico. Dr. Sayed is with the Department of Dermatology at the University of North Carolina School of Medicine in Chapel Hill, North Carolina. Dr. Phadke is with Dermpath Diagnostics South Florida in Pompano Beach, Florida.
FUNDING: No funding was provided for this study.
DISCLOSURES: Dr. Christopher Sayed is a speaker, advisory board member, and investigator for AbbVie; speaker and investigator for Novartis; advisory board member and investigator for UCB; advisory board member and investigator for Inflarx.
ABSTRACT: Galli-Galli disease (GGD) is a rare genodermatosis that is distinguished from Dowling-Degos disease (DDD) by the histologic finding of acantholysis. We present a case of a female patient with pruritic intertriginous plaques and history of hidradenitis suppurativa (HS). While reports exist associating DDD with HS, to our knowledge, GGD in association with HS has not been reported in recent literature. HS in association with DDD has been found to have causal mutations, involving the gamma-secretase complex and POFUT1 genes. DDD also has shared causal mutations with GGD in the POGLUT1 and KRT5 genes. These three skin diseases have been linked to different gene mutations, which are all associated with the Notch signaling pathway.
KEYWORDS: Comorbidity, Dowling-Degos disease (DDD), Galli-Galli disease (GGD), genetics, hidradenitis suppurativa (HS), hyperpigmentation
Galli-Galli disease (GGD) is a rare skin disease that is inherited in an autosomal dominant manner and presents with reticulated, hyperpigmented macules and pruritic, scaly, erythematous papules distributed mainly in flexural regions.1–3 GGD has been previously described as a variant of Dowling-Degos disease (DDD) with the defining histopathological difference of acantholysis.1–3 In addition to their similar clinical and pathological presentation, similar genetic mutations have been linked to their pathogenesis. DDD has also been reported with hidradenitis suppurativa (HS) in patients with mutations in gamma-secretase complex genes.4–6 HS is a chronic inflammatory skin disease that is characterized by the recurrence of inflammatory nodules and abscesses with sinus tract formation and scarring.7,8 Here, we present a patient with concomitant HS and GGD.
Case Report
A 33-year-old African American female patient presented to the University of North Carolina Dermatology clinic with a longstanding history of a pruritic rash primarily located between the breasts and inframammary area. This had been previously treated with triamcinolone 0.1% ointment, but she reported worsening pruritus. She reported a history of abscesses and sinus tract formation in the axillary and groin region but was otherwise healthy with a prior history of smoking. Current medications included ethinylestradiol/etonogestrel vaginal ring and vitamin B12 injections. Family history was negative for similar skin findings. A physical exam demonstrated confluent symmetric hyperpigmentation and thin macerated plaques of the mid central chest extending to the inframammary region (Figure 1). Similar findings with scar and sinus tract formation was present in the axillae, consistent with Hurley Stage II HS (Figure 2).
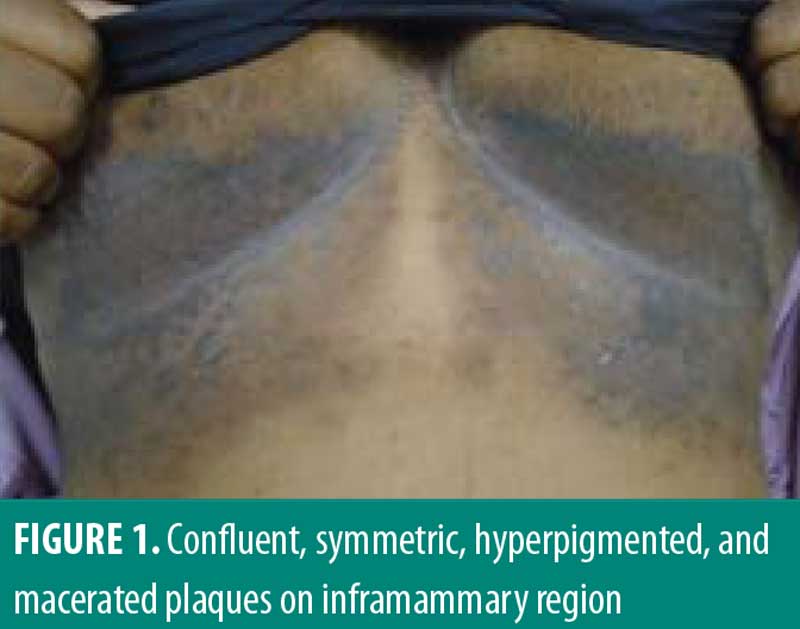
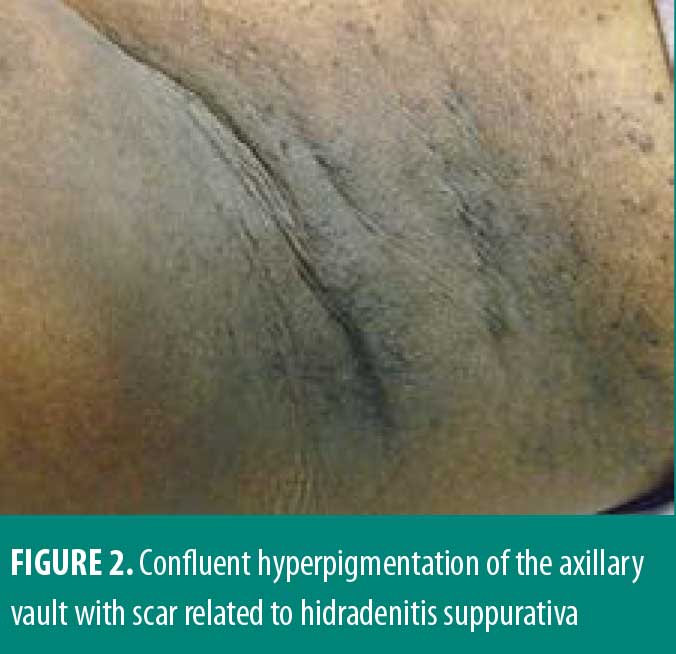
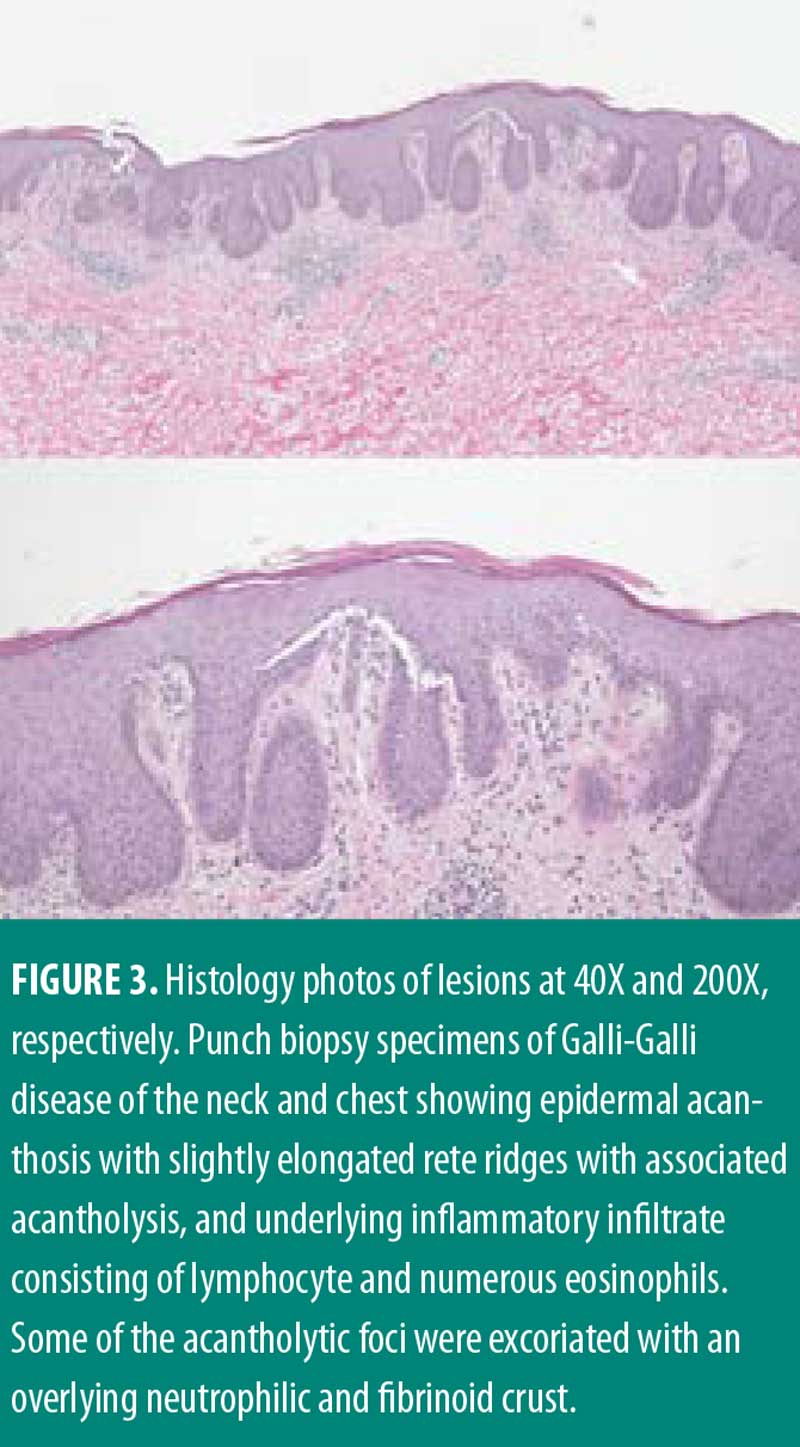
Management for HS included chlorhexidine washes and clindamycin 1% solution applied once daily to the axillae. Biopsies were collected from the right neck and right medial breast. Histologic findings for both biopsies were similar, showing epidermal acanthosis with slightly elongated rete ridges and associated acantholysis (Figures 3 and 4). The acantholytic foci were excoriated with an overlying neutrophilic and fibrinoid crust with scant dermal inflammation consistent with GGD.

Upon follow-up, patient reported getting more frequent flares of her HS in the axilla and groin area. Her GGD was treated with erbium:yttrium-aluminum-garnet (Erb:YAG) laser resurfacing with only modest improvement. Treatment for HS was escalated to clindamycin lotion daily, diclofenac gel up to twice daily, as needed for pain, minocycline 100mg twice daily, and treatment with neodymium:yttrium-aluminum-garnet (Nd:YAG) laser.
Discussion
We presented a clinical link between HS and GGD, which might be explained by shared pathogenic mechanisms. Genetic mutations have created clear links between GGD and DDD, and similar correlations have been reported between HS and DDD. Mutations in the genes KRT5 and POGLUT1 have been reported as causal mutations in both GGD and DDD; while mutations in PSENEN and POFUT1 have been reported in cases of DDD with comorbid HS.4,6,9,10 GGD and HS have been associated to different genetic mutations, but it is in their underlying mechanisms where a connection is found.
The identification of the causal KRT5 gene mutation for DDD by Betz et al11 resulted to be a loss-of-function mutation and one of the few mutations known to cause an alteration of melanosomes in keratinocytes. Their work also led to the description of new important roles for keratins, such as the organization of cell adhesion, melanosome uptake into keratinocytes, organelle transport, and nuclear anchorage.11 These reported abnormalities in keratin function likely translate into the characteristic hyperpigmentation seen in DDD. Basmanav et al12 argued that individuals with KRT5 mutations presented as a DDD phenotype with lesion location typically involving the flexural folds. Reisenauer et al13 clarified that this genotype-phenotype association is also found in epidermolysis bullosa simplex (EBS) with mottled pigmentation, in which a KRT5 mutation in the same domain region affected in DDD and GGD has been observed. The hyperpigmentation seen in each phenotype supports the role of KRT5 in melanosome transport.
The gamma-secretase complex of proteins has been a topic of interest in regard to etiology of DDD, HS, and comorbid HS with DDD.4–6 Of this set of proteins, the presenilin enhancer protein 2 encoded by PSENEN is a subunit of the gamma-secretase complex.4 Through their in vivo studies, Ralser et al4 found that a mutation in PSENEN resulted in a decrease in pigment cells and a variation in the distribution of pigment correlating to clinical features in DDD. In a cohort of patients with comorbid DDD-HS, the histological finding of follicular hyperkeratosis is proposed as a possible identifier of patients with DDD who might be susceptible to HS. Ralser et al4 explained that one of the roles of this encoded protein is the intracellular cleavage of Notch receptors, which is key in Notch signaling activation. While studying DDD-HS, Pavlovsky et al6 found downregulated expression of the PSENEN gene in patient keratinocytes and effector genes of this signaling pathway. They also found this mutation in both a patient with HS and in three patients with HS-DDD and comorbidities, proposing PSENEN and Notch signaling as key in disease pathogenesis.6 Notch signaling has been associated with DDD through other mutations as well.
The protein-O-fucosyltranferase 1 is encoded by the gene POFUT1. This gene has been reported as having a causal role in the development of DDD.9 Of the different types of described genetic variants, only haploinsufficiency was found to lead to changes in skin pigmentation.9 The POFUT1 gene has not only been reported in association with skin pigmentation abnormalities, but with hair pigmentation as well, linking the Notch signaling pathway to melanocyte development and hair pigmentation.14 The role of a loss-of-function mutation in POFUT1 in the Notch signaling pathway was validated by Li et al9 who found downregulated expression of the receptor-encoding genes NOTCH1, NOTCH2, and target gene HES1 in HaCaT cells with silenced POFUT1. Further supporting the pathogenic role of a mutation in POFUT1 via alterations to Notch signaling, Gonzalez-Villanueva et al10 also reported a case of a patient with both DDD and HS that was found to have this mutation.
POGLUT1 is also a regulator of the Notch signaling pathway.9,10,12,15 POGLUT1 encodes the O-glucosyltransferase 1 protein.12,15 Basmanav et al’s study12 findings led to the understanding that POGLUT1 might play a role in the differentiation and development of the epidermis, and that its mutations can lead to abnormal pigmentation and keratinocyte morphology via modification of epidermal growth factor-like repeats of Notch receptors.15 Basmanav et al12 argued that individuals with POGLUT1 mutations presented with a different phenotype, with lesion location mostly involving nonflexural areas, proposing a correlation between the DDD phenotypes and associated genetic variants. Wilson et al15 also found that POGLUT1 mutations at different locations resulted in different phenotypic presentations. They also describe DDD and GGD as a unit through their associated mechanisms in the literature, further supported with their study of patients’ histologic findings and distribution of typical lesions.15
GGD has clinical findings similar to DDD,1,2 such as the hyperpigmentation in the flexural regions, which are linked to reported genetic mutations. The range of associated reported mutations explains the variable patterns of dyspigmentation encountered, and some are likely linked to the finding of follicular occlusion and potentially increased risk of comorbid HS. Both GGD and HS have been reported in association with different mutations, but POFUT1, POGLUT1, and PSENEN, have a shared link in their relationship to the Notch signaling pathway.4,5,6,9,10,12,15
Conclusion
This is a unique case of a patient presenting with both HS and GGD, which expands on previous reports describing the clinical and underlying pathogenic links between HS and DDD. A limitation to our case is that no genetic assay was carried out in our patient to identify previously reported mutations. Although no genetic mutation has been reported to link HS and GGD, based on the presented follicular and epidermal abnormalities and the previously reported mutations in the gamma-secretase complex, this association might be explained by genetic mutations affecting the Notch signaling pathway.
References
- Gilchrist H, Jackson S, Morse L, et al. Galli-Galli disease: a case report with review of the literature. J Am Acad Dermatol. 2008;58(2):299–302.
- Gomes J, Labareda J, Viana I. Galli-Galli disease: a rare acantholytic variant of dowling-degos disease. Case Rep Med. 2011;2011:703257.
- Arnold AW, Kiritsi D, Happle R, et al. Type 1 segmental Galli-Galli disease resulting from a previously unreported keratin 5 mutation. J Invest Dermatol. 2012;132(8):2100–2103.
- Ralser DJ, Basmanav FB, Tafazzoli A, et al. Mutations in ?-secretase subunit–encoding PSENEN underlie Dowling-Degos disease associated with acne inversa. J Clin Invest. 2017;127(4):1485–1490.
- Agut-Busquet E, Gonzalez-Villanueva I, Romani de Gabriel J, et al. Dowling-degos disease and hidradenitis suppurativa. Epidemiological and clinical study of 15 patients and review of the literature. Acta Derm Venereol. 2019;99(10):917–918.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178(2):502–508.
- Shi T, Bai N, Zhang JA, et al. Mutations in the ?-secretase genes PSEN1, PSENEN, and NCSTN in a family with acne inversa. Eur J Dermatol. 2018;28(3):374–376.
- Gasparic J, Theut Riis P, Jemec GB. Recognizing syndromic hidradenitis suppurativa: a review of the literature. J Eur Acad Dermatol Venereol. 2017;31(11):1809–1816.
- Li M, Cheng R, Liang J, et al. Mutations in POFUT1, encoding protein O-fucosyltransferase 1, cause generalized Dowling-Degos disease. Am J Hum Genet. 2013;92(6):895–903.
- González-Villanueva I, Gutiérrez M, Hispán P, et al. Novel POFUT1 mutation associated with hidradenitis suppurativa-Dowling-Degos disease firm up a role for Notch signaling in the pathogenesis of this disorder. Br J Dermatol. 2018;178(4):984–986.
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78(3):510–519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomal-dominant Dowling-Degos disease. Am J Human Genet. 2014;94(1):135–143.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2013;170(6):1362–1365.
- Schouwey L, Delmas V, Larue L, et al. Notch1 and Notch2 receptors influence progressive hair graying in a dose-dependent manner. Dev Dyn. 2007;236(1):282–289.
- Wilson NJ, Cole C, Kroboth K, et al. Mutations in POGLUT1 in Galli-Gali/Dowling-Degos disease. Br J Dermatol. 2017;176(1):270–274.

