 J Clin Aesthet Dermatol. 2020;13(12):29–31.
J Clin Aesthet Dermatol. 2020;13(12):29–31.
by Jessica Shen Tsy Wu Kim, MD; Lilia Ramos dos Santos Guadanhim, MD, PhD; Gisele Jacobino de Barros Nunes, MD; Marco Alexandre Dias da Rocha, PhD; Marco Antonio Munia, MD, PhD; and Samira Yarak, MD, PhD
Drs. Kim, Ramos, Jacobino, Dias da Rocha, and Yarak are with the Department of Dermatology at the Federal University of São Paulo in São Paulo, Brazil. Dr. Munia is with the Department of Vascular Surgery at the University of Sao Paulo in São Paulo, Brazil.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: The use of hyaluronic acid (HA) fillers for facial rejuvenation has grown widely and is now one of the most performed noninvasive cosmetic procedures. Viral infections can occur, albeit rarely. This report describes a 65-year-old female patient with significant fat tissue loss in the malar region who developed herpes zoster after receiving HA filler for facial volumization. We performed volumization with a total of 2mL of HA in one session. Two days after the procedure, the patient began feeling mild pain in the malar region bilaterally and in the right side of the nasolabial fold. Upon physical examination, vesicles and erythema were observed. Due to the possibility of herpes zoster virus (HZV) infection, the patient was treated with valacyclovir. Ultrasonography with arterial and venous Doppler study revealed normal blood flow in the angular artery path and adequate positioning of the filler. After seven days of valacyclovir, the patient had complete resolution of the lesions. Herpes virus reactivation can be caused by direct axon damage by the needle, by tissue manipulation, and by inflammatory reaction. Herpes simplex virus (HSV) is the virus most commonly involved and its incidence does not exceed 1.45 percent of the complication cases, and HZV is even rarer. Reactivation of HZV might mimic tissue ischemia. Ultrasonography is a noninvasive, fast, and useful tool to evaluate vascular impairment and the positioning of the filler.
KEYWORDS: Filler, herpes virus, ischemia, ultrasonography
The use of hyaluronic acid (HA) fillers for facial rejuvenation has grown widely and is now one of the most performed noninvasive cosmetic procedures. The risk of adverse events depends on the expertise of the professional performing the procedure, the product chosen, the technique, the injection plane, the device used, and the patient’s clinical conditions. Complications can be immediate, early, or late, and can include pain, erythema, ecchymosis, edema, and even necrosis and blindness.1–3 Viral infections can occur, albeit rarely, either by reactivation of herpes simplex virus (HSV) or herpes zoster virus (HZV), with few cases described in the literature. This report describes a case of herpes zoster after volumization of the face with HA filler.
Case Report
A healthy 65-year-old female patient with no history of herpes simplex or herpes zoster infection presented with significant fat tissue loss in the malar region. She had been previously treated with injectable HA filler in the nasolabial fold three years earlier with good cosmetic results. We performed volumization with a total of 2mL of HA in one session. One anterior malar bolus (0.3mL/side) and one bolus in the canine fossa (0.2mL/side) were performed with a 27G, 0.5-inch needle, in the supraperiosteal plane, with negative aspiration in all points. The anterior malar region was treated with retroinjection with a 25G, 1.5-inch blunt tip cannula (0.4mL on the right and 0.6mL on the left with fanning technique) (Figure 1). There were no adverse events in the immediate postprocedure period.
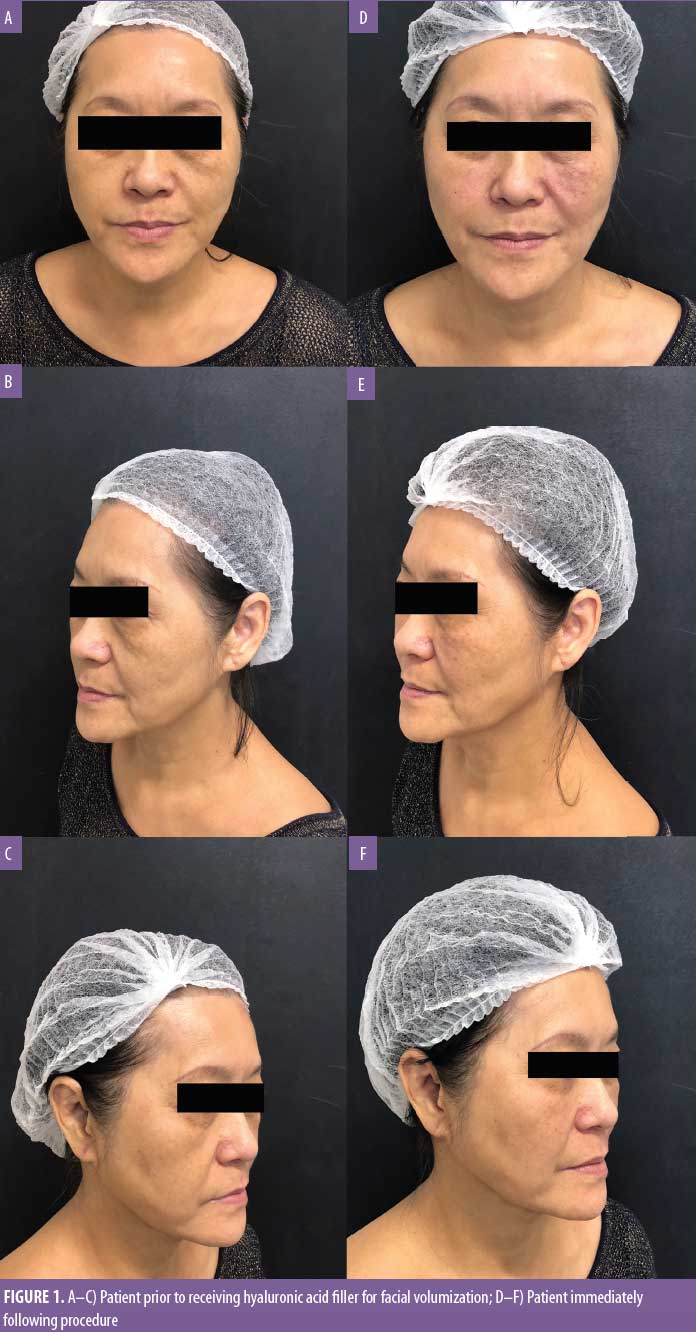
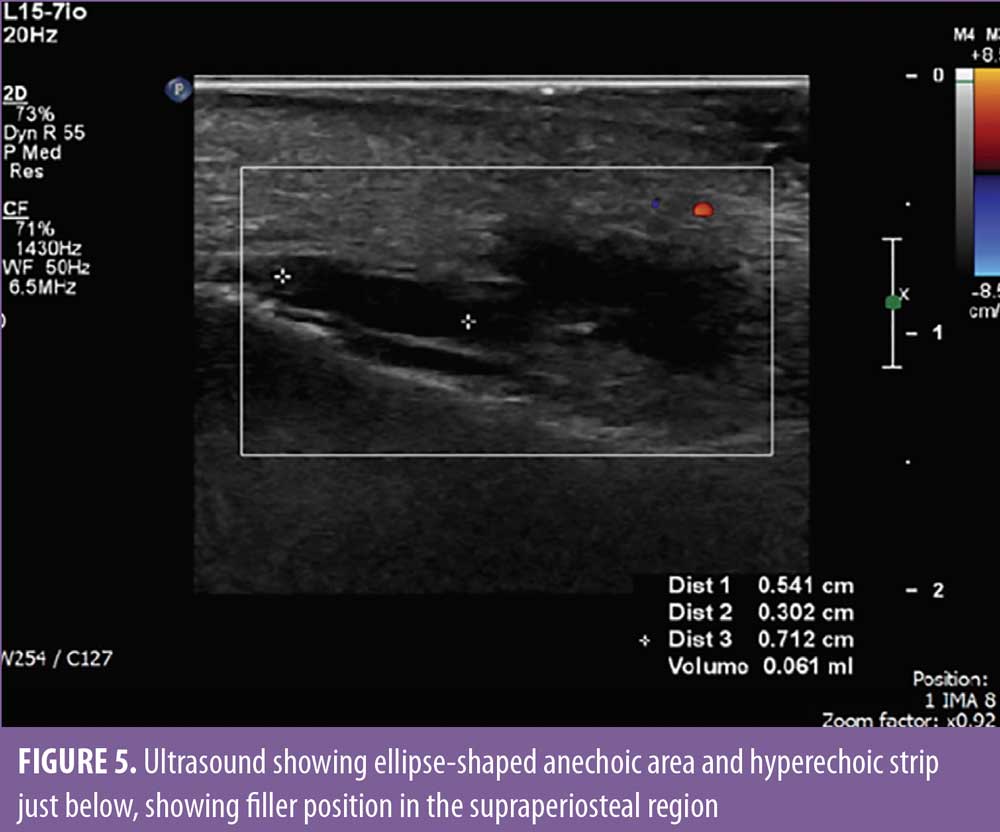
Two days after the procedure, the patient began feeling mild pain in the malar region bilaterally and in the right side of the nasolabial fold, which improved with over-the-counter analgesics. On the fourth day, there was worsening of pain intensity in the malar region, and the patient also complained of temporal headache and shoulder pain, which was more severe on the right side. Upon physical examination, vesicles and erythema were observed in the nasolabial fold and supralabial region on the right (Figures 2 and 3). Due to the possibility of HZV infection, the patient was treated with valacyclovir 1g and analgesia and, as the vesicles and pain arose after the cosmetic procedure, ultrasonography with arterial and venous Doppler study was performed to rule out extrinsic compression and/or intravascular injection. The ultrasound revealed normal blood flow in the angular artery path (Figure 4) and adequate positioning of the filler in the supraperiosteal region (Figure 5), which reinforced the diagnosis of herpes zoster. The patient was also evaluated by an ophthalmologist and there was no ocular involvement by the virus. After seven days of valacyclovir, the patient showed complete resolution of the lesions.



Discussion
Herpes virus reactivation is a rare complication observed following injectable fillers. It can be caused by direct axon damage by the needle, by tissue manipulation, and by inflammatory reaction.4 HSV is the virus most commonly involved, and its incidence does not exceed 1.45 percent of the complication cases, while HZV is even rarer.5,6 Symptoms appear 24 to 48 hours after the procedure. The pattern of reactivation depends on the virus involved and occurs most commonly in the trigeminal ganglion pathway when caused by HSV and ophthalmic branch (V1) of the trigeminal nerve by HZV.6,7 In the case described, the affected branch was the maxillary (V2). Early diagnosis and treatment are critical to avoid complications and sequelae. In the literature, there is only one published case of reactivation of HZV after fillers, related to a calcium hydroxyapatite-based product, and none after HA.4
To differentiate herpes zoster infection from vascular occlusion after a cosmetic procedure using injectables, ultrasound might be useful, especially before the appearance of vesicles, because both could present with erythema and intense pain. Ultrasonography allows visualization of injected HA (hypo-echogenic or anechoic area), as well as evaluation of the injection plane, and Doppler study is able to rule out intravascular injection and extrinsic compression, as shown in the case described.7,8
Conclusion
Reactivation of HZV is a rare complication following the use of HA fillers, and the clinical presentation might mimic tissue ischemia. Ultrasonography is a noninvasive, fast, and useful tool to evaluate vascular impairment and the positioning of the filler
References
- Weinberg M, Solish N. Complications of Hyaluronic Acid Fillers. Facial Plastic Surg. 2009;25(05):324–328.
- Friedman PM, Mafong EA, Kauvar AN, Geronemus RG. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol Surg. 2002;28:491–494.
- Gladstone HB, Cohen JL. Adverse effects when injecting facial fillers. Semin Cutan Med Surg. 2007;26(1):34–39.
- Sires B, Laukaitis S, Whitehouse P. Radiesse-induced herpes zoster. Ophtalmic Plast Reconstr Surg. 2008;24(3):218–219.
- Food and Drug Administration. Restylane Injectable Gel. In: Summary of safety and effectiveness data. 2003.
- Gazzola R, Pasini L, Cavallini M. Herpes virus outbreaks after dermal hyaluronic acid filler injections. Aesthet Surg J. 2012;32(6):770–772.
- King M. Prophylaxis and Treatment of Herpetic Infections. J Clin Aesthet Dermatol. 2017;10(1): E5–E7.
- Micheels P, Besse S, Sarazin D, et al. Ultrasound and histologic examination after subcutaneous injection of two volumizing hyaluronic acid fillers: a preliminary study. Plast Reconstr Surg Glob Open. 2017;5(2):e1222.

