 J Clin Aesthet Dermatol. 2023;16(4):12–20.
J Clin Aesthet Dermatol. 2023;16(4):12–20.
by Etan Marks, DO; Abel Jarell, MD; Joanna Ludzik, MD, PhD; Aaron S. Farberg, MD; Harold S. Rabinovitz, MD; Robert G. Phelps, MD; Clay J. Cockerell, MD; and Alexander Witkowski, MD, PhD
Dr. Marks is with Dermatopathology, Kansas City University-Graduate Medical Education Consortium in Oviedo, Florida, and Advanced Dermatology and Cosmetic Surgery in Oviedo, Florida. Dr. Jarell is with Granite State Dermatology, PC in Portsmouth, New Hampshire. Drs. Ludzik and Witkowski are with the Department of Dermatology, Oregon Health & Science University in Portland, Oregon. Dr. Ludzik is also with the Department of Telemedicine and Bioinformatics, Jagiellonian University Medical College in Krakow, Poland. Dr. Farberg is with Baylor Scott & White Health System in Dallas, Texas. Dr. Rabinovitz is with the Department of Dermatology, Medical College of Georgia in Augusta, Georgia. Dr. Phelps is with Mount Sinai Hospital in New York City, New York. Dr. Cockerell is with Cockerell Dermatopathology in Dallas, Texas and the Departments of Dermatology and Pathology, UT Southwestern Medical Center in Dallas, Texas.
FUNDING: Writing and editorial assistance were funded by Castle Biosciences, Inc.
DISCLOSURES: Drs. Marks, Farberg, and Cockerell have served as consultants and advisory board members for Castle Biosciences, Inc. Dr. Jarell is a speaker, consultant, principal investigator, and advisory board member for Castle Biosciences, Inc. Dr. Rabinovitz has served as an advisory board member for Castle Biosciences, Inc. Dr. Witkowski is a speaker for Castle Biosciences, Inc. Drs. Ludzik and Phelps have no relevant financial conflicts to disclose.
ABSTRACT: Objective. Some melanocytic neoplasms suspicious for melanoma require additional workup to arrive at a final diagnosis. Within the last eight years, gene expression profiling (GEP) has become an important ancillary tool to aid in the diagnosis of melanocytic neoplasms with uncertain malignant potential. As the usage of two commercially available tests (23-GEP and 35-GEP) evolves, it is important to answer key questions about optimal utilization and their impact on patient care.
Methods. Recent and relevant articles answering the following questions were included in the review. First, how do dermatopathologists synthesize the available literature, the latest guidelines, and their clinical experience to determine which cases would be most likely to benefit from GEP testing? Second, how best can a dermatologist convey to their dermatopathologist that the use of GEP in the diagnostic process could provide a more clearly defined result and thereby help empower the dermatologist to provide higher-quality patient care when making specific patient management decisions for otherwise pathologically ambiguous lesions?
Results. When interpreted in the context of the clinical, pathologic, and laboratory information, GEP results can facilitate the rendering of timely, accurate, and definitive diagnoses for melanocytic lesions with otherwise uncertain malignant potential to inform personalized treatment and management plans.
Limitations. This was a narrative review focused on clinical use of GEP compared to other ancillary diagnostic tests performed postbiopsy.
Conclusion. Open communication between dermatopathologists and dermatologists, especially regarding GEP testing, can be a vital component to achieve appropriate clinicopathologic correlation for otherwise ambiguous melanocytic lesions.
Keywords. 23-GEP, 35-GEP, ancillary diagnostic testing, confocal, cutaneous melanoma, dermoscopy, diagnosis, gene expression profile, GEP, histopathology, melanocytic lesions, melanoma.
Gene expression profiling (GEP) utilizes high-throughput, quantitative reverse transcription polymerase chain reaction (qRT-PCR) assayed on custom gene array cards to accurately evaluate the level of ribonucleic acid (RNA) expression of a set of select transcripts.1,2 Typically, about 10 to 50 genes with significant differential expression patterns between disease states are assayed simultaneously.3–6 The gene expression information is used in combination with artificial intelligence-based algorithms to provide an objective test result that can then be utilized with clinicopathological information to inform patient care decisions.2,7
GEP technology has been successfully applied with different gene sets to improve either diagnostic precision or the accuracy of risk prediction for prognostic endpoints (disease relapse/distant metastasis) or predictive endpoints (e.g., likelihood of treatment response) in diverse disease areas, such as thyroid,8 prostate,9 and breast cancers,5 and both uveal3 and cutaneous melanoma.1,2,6 GEP test results provide important clinical information that helps refine patient management and treatment strategies to improve patient outcomes.7,10,11
There are currently two commercial GEP tests available for use as ancillary tools for difficult-to-diagnose cutaneous pigmented lesions: MyPath® Melanoma (23-GEP)1,12–14 and DecisionDx® DiffDx™-Melanoma (35-GEP) (Castle Biosciences; Friendswood, Texas, United States [US]).2 Tissue is obtained from macrodissection of nine slides of formalin-fixed paraffin-embedded (FFPE) tissue, and one additional slide is used for confirmation of the presence of sufficient tissue (at least 10% melanocytic lesion content within a macrodissectible region). Both tests categorize biopsies of melanocytic neoplasms with uncertain malignant potential, with a result of gene expression profile suggestive of benign neoplasm, gene expression profile suggestive of malignant neoplasm, or intermediate gene expression profile cannot exclude malignancy. These results are interpreted in the context of the histopathologic, clinical, and laboratory information for the specific case to aid in the final histopathological assessment and/or to inform appropriate patient management decisions.7,10,11 The GEP tests have demonstrated accuracy metrics of 90.4- to 94.9-percent sensitivity and 92.5- to 96.2-percent specificity for 23-GEP and 94.7- to 99.1-percent sensitivity and 89.5- to 94.3-percent specificity for 35-GEP.1,2,15,16
Here, we describe how to integrate GEP testing into both diagnostic and patient management workflows. This information is intended to guide dermatopathologists and dermatologists alike in the use of GEP testing for melanocytic neoplasms of uncertain malignant potential.
Dermatopathologist-initiated GEP Testing
Hematoxylin and eosin (H&E) staining of FFPE sections for evaluation by light microscopy is the gold standard diagnostic test for all melanocytic lesions. The American Society of Dermatopathology Appropriate Use Criteria (AUC) guidance strongly discourages use of any ancillary test for lesions that are histologically diagnosed as definitively benign nevi or malignant melanomas.17 Ancillary testing, such as GEP, is intended for use in primary cutaneous melanocytic neoplasms with equivocal histologic features or when the malignant potential of the neoplasm is uncertain. Ancillary diagnostic GEP testing is not validated for metastatic melanomas, recurrent tissue specimens, nonmelanocytic neoplasms, or biopsies from patients receiving any type of immunosuppressive therapy or radiation treatment for any medical condition.1,2,15
The most recent guidelines for melanoma released by the National Comprehensive Cancer Network (NCCN) indicate that a variety of available ancillary tests— immunohistochemistry (IHC), array-based comparative genomic hybridization (CGH), fluorescence in situ hybridization (FISH), GEP, single-nucleotide polymorphism (SNP) array, and next-generation sequencing (NGS)—can facilitate diagnosis of ambiguous cases.18 Ancillary tests are not designed or recommended to be used either as a standalone or substitute for routine pathological and clinical evaluation by qualified providers. Nevertheless, the additional information garnered from ancillary testing is a useful tool to be considered contextually with the complete set of clinicopathologic data to aid in personalized clinical decision-making for each patient.
The regulatory assurance for laboratory-based assays is predominantly overseen via the College of American Pathologists (CAP) and the Clinical Laboratory Improvement Act (CLIA), with some additional requirements by local agencies. GEP testing occurs in a central, CAP/CLIA-certified, New York State Department of Health-approved laboratory.19,20 Importantly, GEP testing analytical validity compares favorably with other ancillary tests utilized for difficult-to-diagnose melanocytic neoplasms.21 GEP tests have low technical failure rates of 12.3 to 15.4 percent22–25 for the 23-GEP and 3.4 percent for the 35-GEP.2 IHC for preferentially expressed antigen of melanoma (PRAME), FISH, and other ancillary tools are often utilized without published, individual, laboratory-based metrics and frequently demonstrate interobserver variability.26,27 Additionally, thresholds for positive results may also be inconsistent across laboratories.28,29
GEP testing can be integrated into the diagnostic process by utilization of the test in equivocal melanocytic lesions at various stages. Comments related to the GEP result may be incorporated before a final report is issued and may contain notes related to the impact of the GEP result on the diagnosis. Comments related to the GEP result may also be incorporated after a final report in either an addendum or amended final report. Internal notes may also be used to document GEP usage without the need to modify the final pathology report. If no written comment is made, it could be beneficial for the dermatopathologist to inform the treating dermatologist of the GEP result.
Dermatopathologist Scenarios for GEP Testing
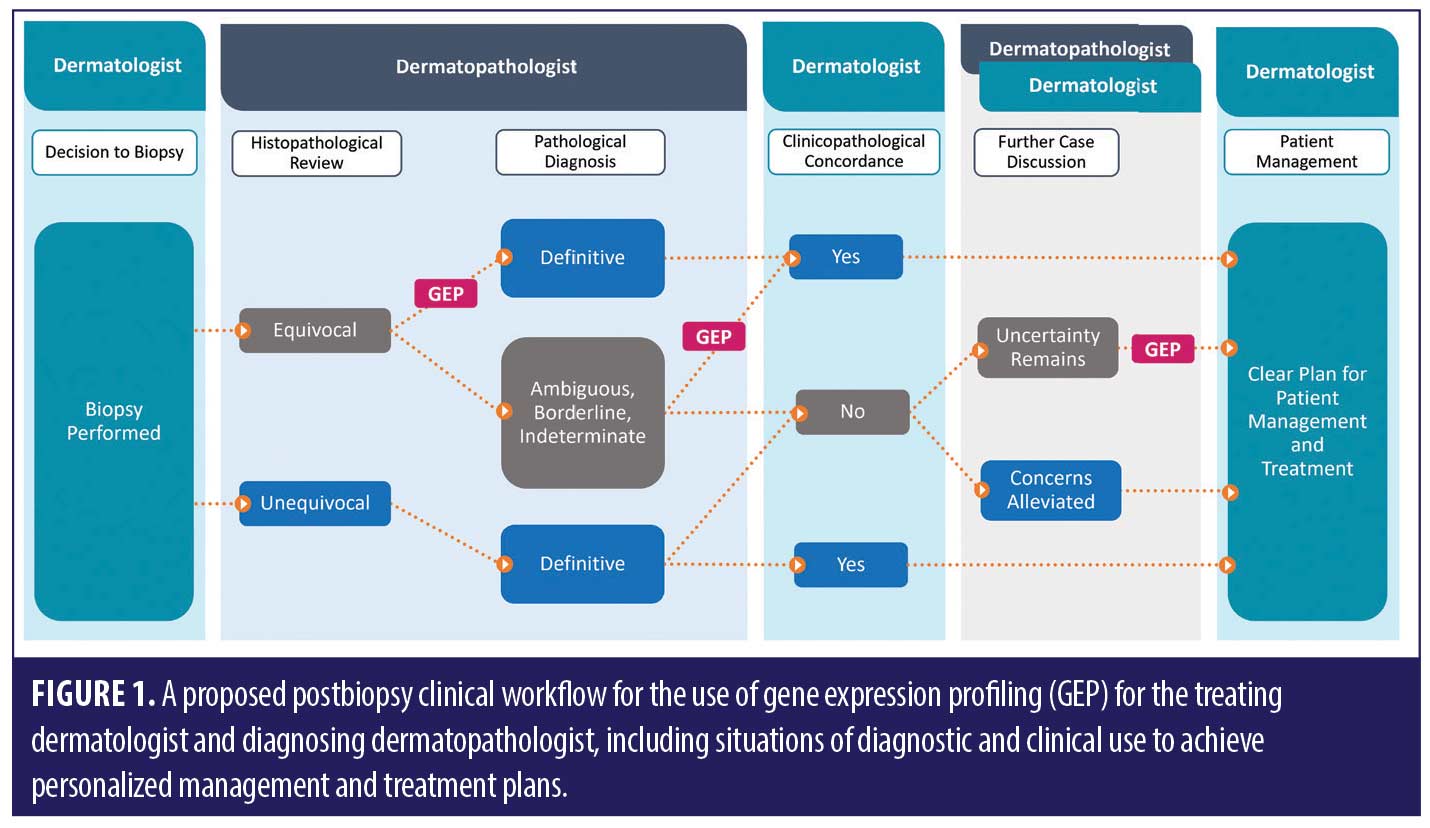
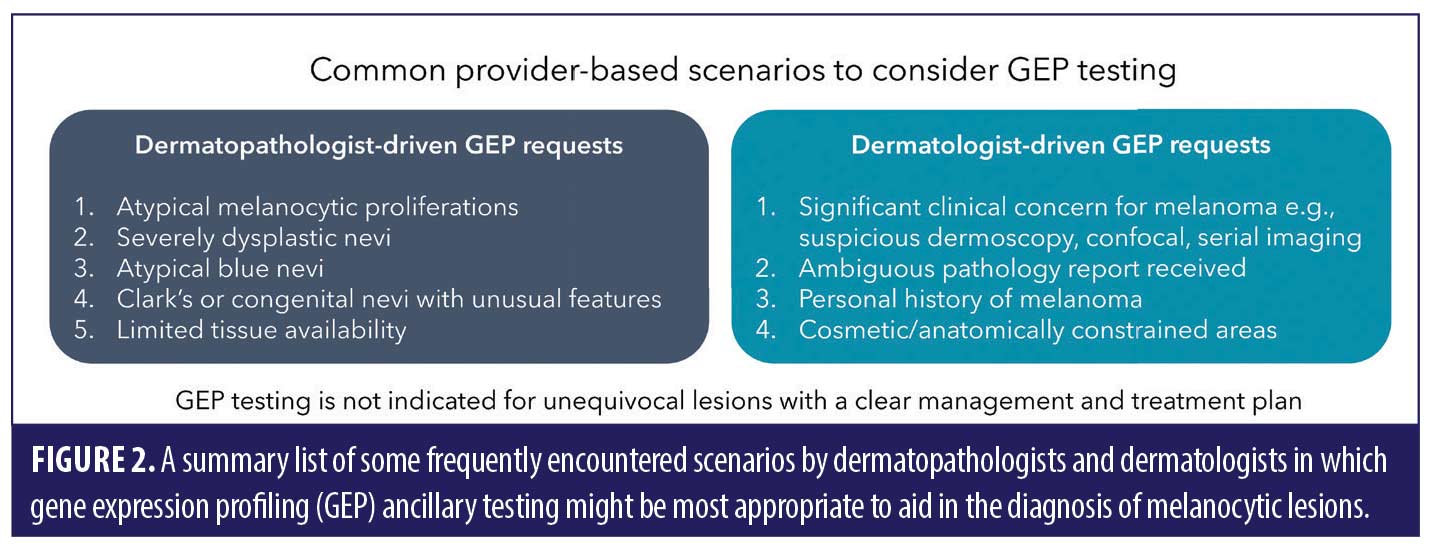
Based on the robust body of scientific evidence available,1,2,7,10,11,13–15,30,31 there are numerous scenarios where GEP can add value for dermatopathologists or pathologists in the process of determining the diagnosis of an equivocal melanocytic lesion (Figure 1).
Atypical melanocytic proliferations and severely dysplastic nevi. The rationale for GEP as an ancillary test in atypical melanocytic proliferations and severely dysplastic nevi is based on the overlapping histopathologic features of nevi and melanoma in these difficult-to-diagnose lesions. In such cases, the histologic diagnosis may remain descriptive and not definitive, which could result in the dermatologist making assumptions about a nondefinitive result and the patient being treated unnecessarily aggressively (e.g., with wide local excision [WLE]). GEP can add clarity to this subset of lesions by identifying malignant risk and increasing the likelihood of a definitive diagnosis. In cases in which the GEP results contradict the preliminary histopathologic diagnosis, the pathologist may consider outside consultation or additional levels to further evaluate the case.
Though FISH testing is reviewed in the current AUC guidelines, PRAME IHC is not.17 Although PRAME staining can sometimes be useful in this subset of lesions, only +4/strongly positive PRAME IHC results can reliably provide high levels of diagnostic confidence in this setting.32 PRAME IHC limitations include false positive and false negative results, lack of internal positive controls in the setting of negative staining, and variable, subjective cutoffs below the +4/strongly positive threshold; all of these factors contribute to the substantial number of lesions that receive inconclusive PRAME results, and these factors are also relevant drawbacks for FISH testing.24,25,33–36 In addition, PRAME IHC and FISH accuracy metrics have previously been published without technical failures and inconclusive results.23 These real-world performance characteristics are important, as other studies have found that technical failures and inconclusive results encompass 10.7 to 11.6 percent of FISH samples and up to 25 percent of PRAME IHC samples (with a 0 to +3 score).24,25,37
Typically, atypical melanocytic proliferations and severely dysplastic nevi fall into the following dermatopathology AUC scenarios, where GEP use is described as “majority usually appropriate:” “Distinction of nevus from primary melanoma in an adult patient when the morphologic findings are ambiguous by light microscopic parameters” and “Distinction of nevus from primary melanoma in an adult patient when the partial nature of the biopsy precludes optimal assessment by light microscopic parameters.”17
Blue nevus-like melanomas, cellular blue nevi, and atypical/malignant blue nevi. Blue nevus-like lesions exist on a continuum and may have overlapping benign and malignant histopathologic features. Evaluation of the degree of atypia, cellularity, and number of mitoses might not always be sufficient for a definitive diagnosis. Interpretation of IHC stains for blue nevi is also constrained where HMB-45 staining pattern, Ki-67/phosphohistone H3 have limited interobserver reliability or published accuracy metrics.38 Similarly, data supporting FISH or CGH for atypical blue nevi are more limited than in other subtypes (e.g., Spitz nevi).39–41 In contrast, GEP testing has shown a 97.8-percent specificity in blue nevi (42/43 cases with a benign result), indicating high performance in this subtype.2 GEP ancillary testing in these ambiguous cases may help clarify the diagnosis of these lesions, potentially decreasing unnecessary excisions and follow-up. The dermatopathology AUC recognize the potential utility of GEP in these lesions by giving the following scenarios a “majority usually appropriate” rating: “Adult patient with pathology suggestive or suspicious for melanoma: atypical blue nevus vs benign blue nevus”and “Adult patient with pathology suggestive or suspicious for melanoma: blue nevus-like melanoma (malignant blue nevus) vs benign blue nevus.”17 In the setting of the limitations of histopathology, IHC, and FISH and in the context of GEP’s high level of accuracy, the potential for diagnostic clarity is high in atypical blue nevi.
Desmoplastic melanoma and benign mimickers. Desmoplastic melanoma is one of the most missed melanoma subtypes due to the bland cytopathology that can histologically mimic benign sclerosing melanocytic neoplasms and even nonmelanocytic desmoplastic neoplasms (e.g., desmoplastic neurofibroma).42 The diagnosis of desmoplastic melanoma therefore requires significant histopathologic experience to accurately identify features concerning for malignancy, confirm the melanocytic origin of the lesion, and exclude other benign nonmelanocytic mimickers.43–45 The wide variety of histopathological presentations suggests a need for ancillary testing, such as GEP, to resolve the differential diagnoses in many cases.
In particular, the 35-GEP has shown high levels of accuracy for desmoplastic melanomas. A preliminary report from a subanalysis of the 35-GEP in 48 desmoplastic melanomas showed a 93.8-percent sensitivity.46 A previous study focusing on the accuracy of the 23-GEP in a cohort of lesions with desmoplastic features found an 80-percent sensitivity for identifying desmoplastic melanomas. The four out of 20 cases misclassified as suggestive of benign were mixed/neurotropic or spindle cell melanoma with desmoplastic features, suggesting that further testing with cases falling among these rare, complex subtypes might be insightful. Building an appropriate benign comparator set of lesions for desmoplastic lesions can be problematic. However, the 23-GEP test showed a 100-percent specificity among lesions, with a benign consensus diagnosis with subtyping ranging from desmoplastic nevi, Spitz nevi (including sclerotic desmoplastic variants), and congenital/conventional nevi with sclerosis.12
While there is evidence that pure desmoplastic melanomas may behave in a less aggressive fashion than mixed desmoplastic melanomas, current American Joint Committee on Cancer (AJCC) staging approaches (8th edition) do not differentiate these subtypes.47,48 A recent study with a small cohort found only 27.3 percent of pure desmoplastic melanomas showed evidence of positive staining with Melan-A/HMB-45, but S100 staining was much more sensitive (13/14 positive staining); only limited evaluation of SOX-10 staining was included in the study.49 In addition, PRAME staining may have inadequate utility, as only about 35 percent of desmoplastic melanomas demonstrate PRAME positivity.35
Additionally, FISH assays for desmoplastic lesions have a lower reported sensitivity in similarly sized studies, ranging from 47 to 63 percent, though FISH testing is generally supported by the AUC.50,51 Accurate analysis of ancillary tests in rare subtypes of melanoma, including desmoplastic lesions, is confounded by relatively high rates of subtyping discordance, even with respect to more basic categories.52 In the absence of specific support for the 23-GEP in the current AUC guidelines (AUC scenario: “Adult patient with pathology suggestive or suspicious for melanoma: sclerosing [desmoplastic] nevus incompletely sampled vs desmoplastic melanoma”17), decisions to use GEP should be based on histologic and clinical features, as well as published accuracy metrics.17 In cases with especially unusual or complex histopathological features, frequent two-way communication between the pathologist and treating physician is critical; the complete set of clinical and pathological information should be weighed in consort with all ancillary test results when deciding on the best course of action (Figure 1).
Spitzoid neoplasms. The rationale for utilization of a specific ancillary test over others in spitzoid neoplasms is less definitive, though GEP testing may be deemed appropriate at any point in the diagnostic workflow. It may even be appropriate to send a lesion for multiple ancillary assays to arrive at a clear diagnosis, while also considering the possibility of disagreement between different test results.23–25,37 Additionally, the rates of assay failure and equivocal/indeterminate/low-positive/borderline results from all ancillary tests have to be considered. IHC stains, such as p16, Ki67, and HMB-45, or FISH can significantly alter the initial impressions from H&E alone.53 PRAME staining has been shown to have somewhat higher false positive rates in Spitz nevi or atypical Spitz tumors.54,55 A recent study of 59 spitzoid melanocytic neoplasms with known clinical outcomes found that false negatives in this subtype class were very common, with diffusely positive PRAME staining identifiable in only 33.3 percent of spitzoid melanomas (and 2.6% of benign Spitz nevi/atypical lesions).56
The AUC guidelines indicate FISH, CGH, and telomerase reverse transcriptase promoter (TERT-p) mutation analysis are “usually appropriate” for “incompletely sampled unclassified Spitz tumor vs spitzoid melanoma” and “atypical Spitz tumor vs spitzoid melanoma” in both pediatric and adult cases.17 However, suboptimal sensitivity rates around 70 percent have been reported for FISH testing in lesions with spitzoid features, indicating a substantial risk of missing FISH-negative melanomas.57 If ancillary testing, such as FISH, is inconclusive or even negative for spitzoid lesions, GEP testing may help to increase the identification of FISH-negative spitzoid melanomas.25 Spitzoid neoplasms have been found to exhibit a wide variety of driver mutations (e.g., HRAS, NRAS, BRAF) or genomic fusions (e.g., ALK, BRAF, RET).58,59 GEP testing has been validated across such a large range of samples that it is able to reliably predict the malignant potential based on gene expression changes that might be common to many melanoma subtypes resulting from numerous genomic lesions.1,2,15 Also, GEP can easily be performed even when tissue/cellularity limitations exclude FISH testing altogether; GEP is validated with as little as 10-percent tumor content from a macrodissectible slide area (not of the entire tissue section, which may often contain a large proportion of normal tissue that does not affect processing). The best pathways to a diagnosis and preferred optimal management strategies are highly variable,60 and the objective results obtained with GEP testing may greatly increase diagnostic clarity.
Cosmetically sensitive/anatomically constrained areas. Excision with wide margins on equivocal lesions is the most cautious approach. However, there are cases where erring on the side of caution might be perceived by the patient to have significant drawbacks. Lesions in cosmetically sensitive or anatomically constrained sites, such as the face or neck, hands, feet, nail beds, or genitals, might benefit from the added information given by GEP testing. Lesions excised from the lips, lower extremities, or ears also have significantly higher rates of infectious complications.61 While the interpreting dermatopathologist or pathologist might not be acutely aware of these body location-specific impacts, the precise anatomic location of a lesion would ideally be readily identifiable on all pathology requisitions. Cases with a malignant GEP result provide clinicians with added confidence regarding the true necessity of excision, making conversations with the patient easier. Those with a benign GEP result could benefit from a narrowing of acceptable surgical margins or may safely forego excision altogether in some cases.31 GEP testing can aid in definitive classification of lesions in these areas and allow for a more appropriate treatment, which might reduce or avoid scar/keloid formation, which can be of particular concern depending on the lesion location. The AUC is supportive of the use of GEP in this scenario as indicated by the ranking of “majority usually appropriate” in the following scenario: “Adult patient with pathology suggestive or suspicious for melanoma: severely atypical compound melanocytic proliferation vs melanoma on cosmetically sensitive areas and special sites, including digits, acral, genital, ears, and scalp.”17
Tissue limitations. GEP testing has the potential to alleviate diagnostic uncertainty in equivocal lesions when limited biopsy tissue is available for testing, whether the biopsy is small, or when tissue must be conserved for other potential testing needs. GEP has a high rate of reproducibility and technical success across a 2,000-fold range of RNA input dilution (down to 2ng/µL).21 Additionally, the tests are validated and have shown consistent accuracy with lesions with a broad range of tumor content, and samples are acceptable for clinical testing as long as there is a macrodissectible area with at least 10-percent melanocytic tumor cells. Biopsies with any of these tissue limitation concerns might be better candidates for GEP than for some other ancillary tests, as thin/low tumor content lesions can be difficult to score with FISH.25 Hybridization array platforms also require substantially more tissue to successfully process (e.g., SNP array, 80ng; CGH, 1.3µg genomic DNA) and a higher tumor to normal cell ratio to definitively identify chromosomal copy number changes.62–64
Pediatric cases. Cutaneous melanoma in pediatric patients is extremely rare, with an incidence of about 2.5 cases per million.65 Pediatric cases account for only about 1 to 4 percent of all melanoma diagnoses,66 and most of these occur after the age of 12 years.65 Lesions seen in children are more frequently spitzoid, a subtype with high diagnostic discordance among experts.67 Diagnosis is further complicated by a general reluctance to diagnose a pediatric patient with melanoma.68,69 Atypical Spitz tumors were shown to have overwhelmingly benign outcomes in a large pediatric retrospective study of 595 patients diagnosed before the age of 20 years.70 Racial disparities noted in a cohort of fatal cases of pediatric cutaneous melanoma suggest rare congenital somatic mutations might play a larger role in this age group.71 While the likelihood of a melanocytic lesion being truly malignant in pediatric patients is relatively low, clinicians have found GEP results useful for uncertain cases, and over 2,000 clinical tests have been performed for pediatric patients with the 23-GEP.72 Careful consideration of the results alongside other clinical information is particularly important and can help preclude unnecessary excisions of lesions with negligible malignant potential. The dermatopathology AUC recognizes the following pediatric scenarios for “majority appropriate use” of GEP testing: “Pediatric patient with pathology suggestive or suspicious for melanoma: nevoid melanoma vs benign melanocytic nevus,” “Pediatric patient with pathology suggestive or suspicious for melanoma: severely atypical compound melanocytic proliferation vs melanoma on cosmetically sensitive areas and special sites, including digits, acral, genital, ears, and scalp,” and “Distinction of nevus from primary melanoma in a pediatric patient when the morphologic findings are ambiguous by light microscopic parameters.”17
Dermatologist Considerations for GEP Testing
There is a management consequence to ambiguity in the pathology final report, which leaves the clinician with uncertainty about how to best manage a patient.73,74 Communication between the dermatologist/treating clinician and the pathologist/dermatopathologist regarding ambiguous pathology reports can alleviate some hesitancy with regard to patient care, and this route of consultation is vital for appropriate patient management.75,76 GEP testing may address this clinically impactful diagnostic uncertainty to better inform management decisions based on the tumor biology of tested lesions.
Even in the context of a relatively clear diagnosis, there can often be significant variability in treatment considerations (e.g., for an atypical junctional nevus with unusual features). Additionally, some of these considerations are based on clinical information that might not have been made available during histopathological review (though ideally would have been clearly stated at the time of biopsy submission). Specifically, the dermatopathologist might not have awareness at the time of diagnosis if (for a particular case) GEP results could aid or change management, including informing follow up, oncology referrals, etc. There could be circumstances when a dermatologist feels it necessary to advocate for their pathology colleague to order GEP for patient management clarifications or send out for the test themselves after review of relevant clinical and pathologic information. Frequent communication, agreement on preferred strategies, and cross-communication of the GEP results are critical, especially for more challenging cases. Regardless of whether the dermatopathologist or dermatologist orders the test, it is important to ensure a shared responsibility for a holistic, balanced interpretation of all information. Figure 1 highlights these instances when clinical concern for diagnostic clarity and persistent uncertainty of malignant potential after final diagnosis could directly impact management decisions and warrant a discussion about the value of including GEP testing.
Dermatologist Scenarios for GEP Testing
There are several scenarios that can highlight the clinical value of GEP in patient management decisions.7,10,11 Dermatologists often have access to more comprehensive clinical information that may not be comprehensively described on a typical requisition form.75,77 There are many cases in which a dermatologist may justify the need for more diagnostic information that can be obtained from GEP testing. These include cases returned with an ambiguous diagnosis, a high degree of cytologic atypia, an architectural disorder that does not meet the interpreting pathologist’s diagnostic threshold for malignancy, concerning dermoscopic and/or reflectance confocal imaging features, or patients with a personal history of melanoma. There are also multiple circumstances as a part of routine practice wherein the pathological diagnosis fails to aid the dermatologist in devising a clear treatment plan, such as atypical intraepidermal melanocytic proliferations (AIMPs) or atypical blue nevi.77,78 These scenarios can lead to the need for clinicians to obtain more information about the lesion at hand to guide treatment decisions and optimize follow-up schedules.
Previous studies have demonstrated clinical use of GEP test results by dermatologists to guide decisions about excision and follow-up frequency.7,10,15,31 Based on this information, GEP results may shift how patients are followed longitudinally for years and adds impetus as to why a definitive diagnosis is crucial for patients. The diagnosis impacts not only primary treatment but also subsequent patient interactions, reducing the amount of gray-zone and subjective interpretation and moving toward a definitive management decision that is agreed upon objectively.
Final pathology report issued with ambiguous diagnosis. If communication regarding ambiguous pathology reports is not sufficient, GEP results can be integrated after receipt of a pathology report. It is important to emphasize that the assimilation of GEP results into the management plans for patients must be conducted in the context of the histopathologic features, laboratory information, and clinical features. In this sense, the same GEP result could have a different clinical impact for a patient depending on the specific nature of the testing situation. For example, in a dysplastic nevus with unusual features, if the GEP result is benign, this could indicate that the patient may benefit from a narrow re-excision instead of a WLE with a margin of normal skin. In the same clinical scenario, if the GEP result is malignant, the test result may be integrated into the diagnostic evaluation, leading to a definitive diagnosis of invasive melanoma or melanoma in situ that would necessitate appropriate patient staging and management decisions. In contrast, an atypical junctional melanocytic proliferation may shift to a recommendation of no excision, in part due to a benign GEP test result, especially if the margins are clear in the initial biopsy; in contrast, re-excision may be recommended after a malignant GEP result.
NCCN guidelines do not currently prioritize any particular ancillary test,18 which might lead to differing opinions about when GEP testing is most appropriate for diagnostic refinement or development of the ideal patient management strategy. Dermatologists are encouraged to readily communicate their desire for GEP testing to their dermatopathologist if they tend to receive final pathology reports with ambiguous diagnoses when GEP has not been considered. However, due to many practical considerations, clinicians often receive reports from multiple pathology professionals who can each have variable individual preferences for ancillary testing workflows. The dermatologist’s lingering, clinically-based uncertainty about the best management strategy could support the dermatopathologist in pursuing GEP testing, which could help clarify the diagnosis in many situations (Figures 1 and 2). However, it also gives the dermatopathologist the opportunity to explain the thought process behind their preferred workups for complicated melanocytic neoplasms, which will allow for better patient care.79
Clinically concerning lesions. Cases often arise with final pathologic diagnosis of benign melanocytic neoplasm; however, significant clinical concern or suspicion of melanoma remains (e.g., cases with long-term sun damage/solar elastosis). For dermatologists trained in dermoscopy evaluation, the presence of a moderate degree of features, such as asymmetry (atypical network), round structures representing lesion growth,80 blue-white discoloration, or other dermoscopic patterns invisible to the naked eye, can also be concerning.81–84 Such scenarios might warrant further evaluation, both by deeper sections of histology and GEP testing to provide further clarification for discordant lesions with clinical features of malignancy yet benign histologic features.
Patient history of melanoma is another important clinical risk factor, both for future development of another melanoma and other skin cancers.85 This level of detail of patient history might not have been communicated to the dermatopathologist. Further assurance that the lesion is benign through GEP testing might be clinically beneficial in such cases. Additionally, a positive GEP result could provide the impetus for additional workup, including deeper sections, which could reveal histological features of melanoma that were not present in the original sections.
In some instances, seemingly contradictory results and recommendations may be included side-by-side in pathology reports; these situations can further compound patient management decisions. For example, a benign diagnosis may be issued with a recommendation to perform re-excision anyway. For lesions in cosmetically sensitive areas, particular and thoughtful care and attention is required,86–88 and dermatologists may seek additional testing with GEP to help inform the final determination as to how wide of margins to re-excise. Again, regular back-and-forth communication between dermatopathologists and all treating clinicians and surgeons involved in patient care is crucial to help resolve misunderstandings and differences of opinion.
Discussion
Melanocytic lesions with unknown malignant potential can be difficult to clinically diagnose and manage because of complex diagnostic scenarios faced by interpreting dermatopathologists or pathologists and subsequent clinical management scenarios faced by treating dermatologists. After the diagnostic biopsy is performed, it is important that all members of the multidisciplinary clinical team are aligned to provide high-quality patient care. The ultimate goals include achieving clinicopathological correlation and personalized treatment plans that avoid missed malignancies and limit unnecessary re-excisions of ultimately benign neoplasms. By simultaneously measuring the expression levels of many genes, GEP technology can identify RNA-level alterations common to a variety of malignant melanocytic lesions that go beyond visible cytologic and architectural changes. Utilization of GEP at various stages, including prediagnosis to achieve diagnostic clarity and postdiagnosis to assuage diagnostic ambiguity and/or alleviate strong clinical suspicion for melanoma in an otherwise histologically unequivocal lesion, can benefit patients by ensuring that the highest level of clinicopathological correlation is reached (Figure 1).
Recent reports suggest that a rise in melanoma overdiagnosis may be, in part, responsible for the observed increase in melanoma incidence over the last few decades.89 One study examining disparity in trends of rising melanoma incidence between White and Black patients estimated that up to 60 percent of White patients thought to have melanoma might have been overdiagnosed.90 Many dermatopathologists surveyed in another study agreed that atypical nevi, melanoma in situ, and/or malignant melanoma are presently overdiagnosed.91 Regardless of the factors at play, these trends further strengthen the argument for GEP testing to help increase the likelihood of a definitive diagnosis and reduce overdiagnosis by increasing diagnostic confidence and accuracy. GEP testing has been predictively shown to reduce healthcare economic burden, in large part due to the reduced number of melanomas misdiagnosed as benign.92 In addition, clinical utility studies focusing on decision points for dermatologists and dermatopathologists with both clinically available GEP tests have consistently shown that a benign test result brings a substantial de-escalation of management intensity, both in treatment/follow-up recommendations and actual rate of excisions performed.7,10,11
When compared to other diagnostic ancillary tools, it is important to highlight the large GEP-tested cohorts that have demonstrated consistent accuracy across two tests using different gene sets.1,2 Several of these genes have subsequently been identified as excellent discriminant genes by multiple independent research groups (e.g., CXCL and S100A family genes).93 GEP validation cohorts included 437 to 860 patients in each of three independent studies and included a wide variety of lesions.1,2,15 The accuracy metrics in these cohorts were about 90 percent or greater. Further studies with the 23-GEP have gone beyond histopathological consensus diagnosis among multiple expert dermatopathological reviewers and shown excellent GEP test performance based on long-term patient outcomes.13,14,30,31 It is also important to recognize both the strengths and limitations of other ancillary tests (e.g., IHC, FISH, NGS, SNP/CGH array) and critically evaluate the utility of these assays for each patient.
As discussed in the current AUC, commercial adoption and guidelines often lag behind published evidence for ancillary testing related to the diagnosis of melanocytic neoplasms.17 Though the recommendations are based upon 23-GEP studies (the data analysis cutoff was mid-2019),17 published evidence demonstrates similar accuracy and utility of the 35-GEP test across most subsets of lesions.2,7 Dermatopathologists should consider using GEP in AUC scenarios deemed “majority usually appropriate.” In addition, there are some diagnostic scenarios deemed “uncertain appropriateness”17 in which the use of GEP tests can inform specific patient management decisions and are clincally informative, while ongoing studies will continue to add more clarifying evidence.
Conclusion
Overall, greater communication within the multidisciplinary team—the pathology lab, dermatology clinic, and patients themselves—is the best approach to optimize strategies for melanocytic lesions of unknown malignant potential. This has become more important than ever, given the rapid increase in the number of skin biopsies performed by nonphysician providers, especially in medically underserved areas with limited access to dermatology services.94 Increased access to noninvasive, in-vivo imaging modalities provided by trained specialists will further help to identify suspicious melanocytic neoplasms. GEP testing can aid with diagnosis and clinical decision making for dermatopathologists and dermatologists for atypical melanocytic neoplasms in which histopathological evaluation is insufficient to confidently render an actionable diagnosis or suspicion of malignancy remains due to concerning clinical features.
Acknowledgements
The authors wish to acknowledge Jason H. Rogers, MSc; Brooke Russell, PhD; and Matthew S. Goldberg, MD, of Castle Biosciences, Inc., for writing and editorial assistance.
References
- Clarke LE, Warf MB, Flake DD, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42(4):244–252.
- Estrada S, Shackelton J, Cleaver N, et al. Development and validation of a diagnostic 35-gene expression profile test for ambiguous or difficult-to-diagnose suspicious pigmented skin lesions. SKIN. 2020;4(6):506–522.
- Onken MD, Worley LA, Tuscan MD, Harbour JW. An accurate, clinically feasible multi-gene expression assay for predicting metastasis in uveal melanoma. J Mol Diagn. 2010;12(4):461–468.
- Litchman GH, Fitzgerald AL, Kurley SJ, et al. Impact of a prognostic 40-gene expression profiling test on clinical management decisions for high-risk cutaneous squamous cell carcinoma. Curr Med Res Opin. 2020;36(8):1295–1300.
- Cobleigh MA, Tabesh B, Bitterman P, et al. Tumor gene expression and prognosis in breast cancer patients with 10 or more positive lymph nodes. Clin Cancer Res. 2005;11(24):8623–8631.
- Gerami P, Cook RW, Russell MC, Wilkinson J, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72(5):780–785.e3.
- Farberg A, Ahmed K, Bailey C, et al. A 35-gene expression profile test for use in suspicious pigmented lesions impacts clinical management decisions of dermatopathologists and dermatologists. SKIN. 2020;4(6):523–533.
- Krane JF, Cibas ES, Endo M, et al. The Afirma Xpression Atlas for thyroid nodules and thyroid cancer metastases: insights to inform clinical decision-making from a fine-needle aspiration sample. Cancer Cytopathol. 2020;128(7):452–459.
- Cuzick J, Swanson GP, Fisher G, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–255.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95(40):e4887.
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14(2):123–130.
- Clarke LE, Pimentel JD, Zalaznick H, et al. Gene expression signature as an ancillary method in the diagnosis of desmoplastic melanoma. Hum Pathol. 2017;70:113–120.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26(7):1107–1113.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213–221.
- Clarke LE, Flake DD, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123(4):617–628.
- Goldberg M, Siegel J, Russell B, et al. A comprehensive diagnostic offering workflow increases the rate of actionable results of the 23- and 35-gene expression profile tests for use as ancillary diagnostic tools for difficult-to-diagnose melanocytic lesions. J Skin. 2021;5(6):s79.
- AUC Committee Members, Fung MA, Vidal CI, et al. Appropriate use criteria for ancillary diagnostic testing in dermatopathology: New recommendations for 11 tests and 220 clinical scenarios from the American Society of Dermatopathology Appropriate Use Criteria Committee. J Cutan Pathol. 2022;49(3):231–245.
- National Comprehensive Cancer. NCCN Guidelines Melanoma: Cutaneous Version 2.2022. National Comprehensive Cancer Network; 2022.
- Sun F, Bruening W, Uhl S, et al. Quality, Regulation and Clinical Utility of Laboratory-developed Molecular Tests. Agency for Healthcare Research and Quality (US); 2010.
- Engstrom PF, Bloom MG, Demetri GD, et al. NCCN molecular testing white paper: effectiveness, efficiency, and reimbursement. J Natl Compr Canc Netw. 2011 Dec;9 Suppl 6:S1–S16.
- Warf MB, Flake DD, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9(5):407–416.
- Reimann JDR, Salim S, Velazquez EF, et al. Comparison of melanoma gene expression score with histopathology, fluorescence in situ hybridization, and SNP array for the classification of melanocytic neoplasms. Mod Pathol. 2018;31(11):1733–1743.
- Clarke LE, Leachman SA. Reply to Reimann et al. Mod Pathol. 2019;32(5):725–727.
- Castillo SA, Pham AK, Dagrosa AT, et al. Concordance analysis of the 23-gene expression signature (myPath Melanoma) with fluorescence in situ hybridization assay and single nucleotide polymorphism array in the analysis of challenging melanocytic lesions: results from an academic medical center. Am J Dermatopathol. 2020;42(12):939–947.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29(8):832–843.
- Gerami P, Mafee M, Lurtsbarapa T, et al. Sensitivity of fluorescence in situ hybridization for melanoma diagnosis using RREB1, MYB, Cep6, and 11q13 probes in melanoma subtypes. Arch Dermatol. 2010;146(3):273–278.
- Saleem A, Narala S, Raghavan SS. Immunohistochemistry in melanocytic lesions: updates with a practical review for pathologists. Semin Diagn Pathol. 2022;39(4):239–247.
- McCalmont TH. Fillet of FISH. J Cutan Pathol. 2011;38(4):327–328.
- Ferrara G, Vanna ACD. Fluorescence in situ hybridization for melanoma diagnosis: a review and a reappraisal. Am J Dermatopathol. 2016;38(4):253–269.
- Clarke LE, Mabey B, Flake II DD, et al. Clinical validity of a gene expression signature in diagnostically uncertain neoplasms. Per Med. 2020;17(5):361–371.
- Tschen J, Davies P, Meek SM, Clarke LE. Clinical use of a diagnostic gene expression signature for melanocytic neoplasms. Cutis. 2021;107(5):264–269.
- O’Connor MK, Dai H, Fraga GR. PRAME immunohistochemistry for melanoma diagnosis: a STARD compliant diagnostic accuracy study. J Cutan Pathol. 2022; 49(9):780–786.
- Kline N, Menge TD, Hrycaj SM, et al. PRAME expression in challenging dermal melanocytic neoplasms and soft tissue tumors with melanocytic differentiation. Am J Dermatopathol. 2022;44(6):404–410.
- Fattori A, de la Fouchardière A, Cribier B, Mitcov M. Preferentially expressed Antigen in MElanoma immunohistochemistry as an adjunct for evaluating ambiguous melanocytic proliferation. Hum Pathol. 2022;121:19–28.
- Lezcano C, Jungbluth AA, Busam KJ. PRAME immunohistochemistry as an ancillary test for the assessment of melanocytic lesions. Surg Pathol Clin. 2021;14(2):165–175.
- Lezcano C, Jungbluth AA, Busam KJ. Comparison of immunohistochemistry for PRAME with cytogenetic test results in the evaluation of challenging melanocytic tumors. Am J Surg Pathol. 2020;44(7):893–900.
- Harvey NT, Peverall J, Acott N, et al. Correlation of FISH and PRAME immunohistochemistry in ambiguous superficial cutaneous melanocytic proliferations. Am J Dermatopathol. 2021;43(12):913–920.
- Compton LA, Murphy GF, Lian CG. Diagnostic immunohistochemistry in cutaneous neoplasia: an update. Dermatopathology (Basel). 2015;2(1):15–42.
- North JP, Garrido MC, Kolaitis NA, et al. Fluorescence in situ hybridization as an ancillary tool in the diagnosis of ambiguous melanocytic neoplasms: a review of 804 cases. Am J Surg Pathol. 2014;38(6):824–831.
- Gammon B, Beilfuss B, Guitart J, et al. Fluorescence in situ hybridization for distinguishing cellular blue nevi from blue nevus-like melanoma. J Cutan Pathol. 2011;38(4):335–341.
- Barnhill RL, Argenyi Z, Berwick M, et al. Atypical cellular blue nevi (cellular blue nevi with atypical features): lack of consensus for diagnosis and distinction from cellular blue nevi and malignant melanoma (“malignant blue nevus”). Am J Surg Pathol. 2008;32(1):36–44.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68(5):825–833.
- Cadwell CR, Yuksek GE, Hirbe AC, et al. Preferentially expressed antigen in melanoma (PRAME) expression in malignant, but not benign, peripheral nerve sheath tumors. J Neuropathol Exp Neurol. 2021;80(4):384–386.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35(5):433–444.
- Frydenlund N, Mahalingam M. Desmoplastic melanoma, neurotropism, and neurotrophin receptors–what we know and what we do not. Adv Anat Pathol. 2015;22(4):227–241.
- Hosler GA, Goldberg MS. Performance of 35-gene expression profile test in desmoplastic melanoma. Am J Dermatopathol. 2021;43:S16.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492.
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99.
- Chu S, Schrom KP, Tripathi R, et al. Pure and mixed desmoplastic melanomas: a retrospective clinicopathologic comparison of 33 cases. Am J Dermatopathol. 2021;43(11):776–780.
- Weissinger SE, Frick M, Möller P, et al. Performance testing of RREB1, MYB, and CCND1 fluorescence in situ hybridization in spindle-cell and desmoplastic melanoma argues for a two-step test algorithm. Int J Surg Pathol. 2017;25(2):148–157.
- Gerami P, Beilfuss B, Haghighat Z, . Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38(4):329–334.
- Yardman-Frank JM, Bronner B, Rosso S, et al. Comparison of community pathologists with expert dermatopathologists evaluating Breslow thickness and histopathologic subtype in a large international population-based study of melanoma. JAAD Int. 2021;4:25–27.
- Puri PK, Ferringer TC, Tyler WB, et al. Statistical analysis of the concordance of immunohistochemical stains with the final diagnosis in spitzoid neoplasms. Am J Dermatopathol. 2011;33(1):72–77.
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47(12):1123–1131.
- Koh SS, Lau SK, Scapa JV, Cassarino DS. PRAME immunohistochemistry of spitzoid neoplasms. J Cutan Pathol. 2022;49(8):709–716.
- Gerami P, Benton S, Zhao J, et al. PRAME expression correlates with genomic aberration and malignant diagnosis of spitzoid melanocytic neoplasms. Am J Dermatopathol. 2022;44(8):575–580.
- Cho-Vega JH. A diagnostic algorithm for atypical spitzoid tumors: guidelines for immunohistochemical and molecular assessment. Mod Pathol. 2016;29(7):656–670.
- Elder DE, Bastian BC, Cree IA, et al. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med. 2020;144(4):500–522.
- Benton S, Zhao J, Zhang B, et al. Impact of next-generation sequencing on interobserver agreement and diagnosis of spitzoid neoplasms. Am J Surg Pathol. 2021;45(12):1597–1605.
- Zhao G, Lee KC, Peacock S, et al. The utilization of spitz-related nomenclature in the histological interpretation of cutaneous melanocytic lesions by practicing pathologists: results from the M-Path study. J Cutan Pathol. 2017;44(1):5–14.
- Schlager JG, Ruiz San Jose V, Patzer K, et al. Are specific body sites prone for wound infection after skin surgery? a systematic review and meta-analysis. Dermatol Surg. 2022;48(4):406–410.
- Wang L, Rao M, Fang Y, et al. A genome-wide high-resolution array-CGH analysis of cutaneous melanoma and comparison of array-CGH to FISH in diagnostic evaluation. J Mol Diagn. 2013;15(5):581–591.
- Carter MD, Durham AB, Miedema JR, et al. Molecular testing of borderline cutaneous melanocytic lesions: SNP array is more sensitive and specific than FISH. Hum Pathol. 2019;86:115–123.
- Leachman SA, Mengden Koon S, Korcheva VB, White KP. Assessing genetic expression profiles in melanoma diagnosis. Dermatol Clin. 2017;35(4):537–544.
- Dean PH, Bucevska M, Strahlendorf C, Verchere C. Pediatric melanoma: a 35-year population-based review. Plast Reconstr Surg Glob Open. 2017;5(3):e1252.
- Mccormack L, Hawryluk EB. Pediatric melanoma update. G Ital Dermatol Venereol. 2018;153(5):707–715.
- Gerami P, Busam K, Cochran A, et al. Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up: Am J Surg Pathol. 2014;38(7):934–940.
- Reed D, Kudchadkar R, Zager JS, et al. Controversies in the evaluation and management of atypical melanocytic proliferations in children, adolescents, and young adults. J Natl Compr Canc Netw. 2013;11(6):679–686.
- Enos T, Hughes C, Kelley S, et al. Assessing comfort level with pediatric skin specimens among dermatopathologists and pediatric pathologists: a national cross-sectional survey. J Cutan Pathol. 2021;48(9):1109–1114.
- Bartenstein DW, Fisher JM, Stamoulis C, et al. Clinical features and outcomes of spitzoid proliferations in children and adolescents. Br J Dermatol. 2019;181(2):366–372.
- Hawryluk EB, Moustafa D, Bartenstein D, et al. A retrospective multicenter study of fatal pediatric melanoma. J Am Acad Dermatol. 2020;83(5):1274–1281.
- De La O JP, Mabey B, Flake D, et al. Characteristics of pediatric and adult melanocytic lesions sent for commercial gene expression testing. In Poster Session 1; 2020:294. https://cdn.preciscentral.com/eventdata/P1089/ASDP20Poster294.pdf.
- Patrawala S, Maley A, Greskovich C, et al. Discordance of histopathologic parameters in cutaneous melanoma: clinical implications. J Am Acad Dermatol. 2016;74(1):75–80.
- Taylor L, Hood K, Reisch L, et al. Influence of variability in assessment of Breslow thickness, mitotic rate and ulceration among US pathologists interpreting invasive melanoma, for the purpose of AJCC staging. J Cutan Pathol. 2018;45(8):588–596.
- Smith SDB, Reimann JDR, Horn TD. Communication between dermatologists and dermatopathologists via the pathology requisition: opportunities to improve patient care. JAMA Dermatol. 2021;157(9):1033–1034.
- Korbl JD, Wood BA, Harvey NT. ‘Why don’t they ever call?’ Expectations of clinicians and pathologists regarding the communication of critical diagnoses in dermatopathology. Pathology. 2018;50(3):305–312.
- Cockerell CJ. Commentary on atypical melanocytic proliferations. Dermatol Surg. 2018;44(2):175–176.
- Ensslin CJ, Hibler BP, Lee EH, et al. Atypical melanocytic proliferations: a review of the literature. Dermatol Surg. 2018;44(2):159–174.
- Comfere NI, Peters MS, Jenkins S, et al. Dermatopathologists’ concerns and challenges with clinical information in the skin biopsy requisition form: a mixed methods study. J Cutan Pathol. 2015;42(5):333–345.
- Beer J, Xu L, Tschandl P, Kittler H. Growth rate of melanoma in vivo and correlation with dermatoscopic and dermatopathologic findings. Dermatol Pract Concept. 2011;1(1):59–67.
- Holmes GA, Vassantachart JM, Limone BA, et al. Using dermoscopy to identify melanoma and improve diagnostic discrimination. Fed Pract. 2018;35(Suppl 4):S39–S49.
- Emiroglu N, Cengiz FP, Hofmann-Wellenhof R. Dermoscopic and clinical features of trunk melanomas. Postepy Dermatol Alergol. 2014;31(6):362–367.
- Kato J, Horimoto K, Sato S, et al. Dermoscopy of melanoma and non-melanoma skin cancers. Front Med (Lausanne). 2019;6:180.
- Zalaudek I, Argenziano G, Soyer HP, et al. Three-point checklist of dermoscopy: an open internet study. Br J Dermatol. 2006;154(3):431–437.
- Wei EX, Li X, Nan H. Having a first-degree relative with melanoma increases lifetime risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. J Am Acad Dermatol. 2019;81(2):489–499.
- Magro CM, Crowson AN, Mihm MC, et al. The dermal-based borderline melanocytic tumor: a categorical approach. J Am Acad of Dermatol. 2010;62(3):469–479.
- Zembowicz A, Scolyer RA. Nevus/melanocytoma/melanoma: an emerging paradigm for classification of melanocytic neoplasms? Arch Pathol Lab Med. 2011;135(3):300–306.
- Elder DE, Xu X. The approach to the patient with a difficult melanocytic lesion. Pathology. 2004;36(5):428–434.
- Kurtansky NR, Dusza SW, Halpern AC, et al. An epidemiologic analysis of melanoma overdiagnosis in the United States, 1975-2017. J Invest Dermatol. 2022;142(7):1804–1811.e6.
- Adamson AS, Suarez EA, Welch HG. Estimating overdiagnosis of melanoma using trends among Black and White patients in the US. JAMA Dermatol. 2022;158(4):426–431.
- Kerr KF, Eguchi MM, Piepkorn MW, et al. Dermatopathologist perceptions of overdiagnosis of melanocytic skin lesions and association with diagnostic behaviors. JAMA Dermatol. 2022;158(6):675–679.
- Cassarino DS, Lewine N, Cole D, et al. Budget impact analysis of a novel gene expression assay for the diagnosis of malignant melanoma. J Med Econ. 2014;17(11):782–791.
- Borden ES, Adams AC, Buetow KH, et al. Shared gene expression and immune pathway changes associated with progression from nevi to melanoma. Cancers (Basel). 2022 ;14(1):3.
- Patel S, Nguyen BT. Characterization of biopsies by dermatologists and nonphysician providers in the Medicare population: a rapidly changing landscape. Dermatol Surg. 2021;47(10):1337–1341.

