 J Clin Aesthet Dermatol. 2022;15(11):69–74.
J Clin Aesthet Dermatol. 2022;15(11):69–74.
by James Q. Del Rosso, DO; Jean Philippe York, PhD; and Neal Bhatia, MD
Dr. Del Rosso is with JDR Dermatology Research and Thomas Dermatology in Las Vegas, Nevada, and Advanced Dermatology and Cosmetic Surgery in Maitland, Florida. Dr. York is with Galderma Laboratories, L.P. in Dallas, Texas. Dr. Bhatia is with Therapeutics Clinical Research in San Diego, California.
ABSTRACT: Objective. Subantibiotic dose doxycycline (SDD40), formulated as a modified-release 40mg capsule administered once daily, is used to treat inflammatory lesions of rosacea. In order to investigate whether the patient’s weight or lesion severity impacts clinical outcomes with using SDD40, the efficacy and safety of SDD40 in treating rosacea were evaluated in randomized controlled studies (RCTs).
Methods. Phase II, III, and IV RCTs, and a subsequent meta-analysis were described. For all studies, the primary efficacy endpoint was the change in total inflammatory lesion count (papules, pustules, and nodules) from baseline to Week 16. For one of the studies, body weights were categorized by BMI (body mass index). Secondary efficacy endpoints included the change in Investigator’s Global Assessment (IGA). Safety was assessed by monitoring adverse events (AEs).
Results. The efficacy of SDD40 was consistent across the studies (two trials including n=72 and n=91 subjects) and meta-analysis (n=127 and n=142). SDD40 remained effective regardless of baseline disease severity and weight (with a weak correlation coefficient below 0.75); overweight or obese subjects with severe rosacea cleared at least as well if not better than those with a normal BMI and mild disease. The treatment was well tolerated with no to minimal gastrointestinal-related AEs.
Limitations. Retrospective analyses have methodological limitations.
Conclusion. Consistency between study results including the meta-analysis supports the effectiveness and safety of SDD40, irrespective of the weight of the patient or rosacea severity based on inflammatory lesion count at baseline.
Keywords. inflammatory lesions of rosacea, subantimicrobial-dose oral doxycycline, subantibiotic dose oral doxycycline (SDD40), obesity, body mass index (BMI), antimicrobial resistance
Rosacea, a highly prevalent, chronic, fluctuating, inflammatory facial dermatosis,1 may be progressive in severity if left untreated.2 While the pathophysiology is not fully known, its visible manifestations are well-described. Rosacea is reportedly associated with multiple comorbidities in a severity-dependent manner3 and can adversely affect quality of life.4 An increased risk of rosacea is also observed with elevated body mass index (BMI) in a weight-dependent manner according to a prospective cohort study.5 This finding is also supported by a twin study correlating BMI with rosacea.6 It is suggested that clinicians consider a broad range of factors beyond just cutaneous manifestations.
Subantibiotic dose oral doxycycline, previously referred to as subantimicrobial and anti-inflammatory dose in the literature (40mg modified release capsules [30mg immediate-release, 10mg delayed-release beads], hereafter called SDD40), is the only Food and Drug Administration (FDA)-approved oral therapy for inflammatory lesions of rosacea. It has been successfully combined with topical metronidazole,7 topical ivermectin,8 or topical azelaic acid9 in clinical trials. Plasma concentrations of SDD40 remain below the threshold necessary for antibiotic activity10 while exerting anti-inflammatory activity such as decreasing cathelicidin and protease activity, which may be predictors of clinical response.11
Doxycycline may counter the inflammation in rosacea via modulation of several factors, including serine proteases, matrix metalloproteinases, chemotaxis, granuloma formation, and reactive oxygen species.12 The anti-inflammatory activity of SDD40 is also demonstrated by effectively and safely reducing inflammatory lesions of rosacea in two large randomized clinical trials.13 In patients with rosacea of mainly moderate severity treated with topical metronidazole and either SDD40 or antibiotic-dose doxycycline 100mg daily, therapeutic response was nearly identical yet gastrointestinal (GI) side effects were noted in the antibiotic-dose group.14 Furthermore, in a cross-sectional study from the Humana claims database among adults with a history of rosacea, researchers hypothesized that the association found between GI disease and rosacea is at least partly mediated by doxycycline use. They found that more GI diseases were associated with conventional, immediate-release formulations of doxycycline with antibiotic activity (50 or 100mg) than with SDD40.15 Also, no significant difference was seen in the prevalence of GI diseases between patients taking SDD40 and those not taking doxycycline. Although causality could not be determined, these results suggest that antibiotic use with doxycycline (at 50 or 100mg), not necessarily rosacea itself, may contribute to this association.
In a first regression analysis of two 16-week Phase III studies, no dose-efficacy relationship was found for SDD40 at any visit in terms of mean change from baseline in inflammatory lesion counts (Figure 1).16 This supports that overall, SDD40 provides optimized efficacy. In the real world though, clinicians may use variable doses of oral antibiotics for rosacea. A common approach involves starting at a higher dose in an attempt to achieve control, then tapering down for a “maintenance dose,” despite lack of confirmatory evidence for this approach. Alternatively, based on evidence corroborating similar efficacy at lower doses compared to higher doses and for patients with lower body weight,17 some clinicians prefer to start treatment at a low daily dose. However, some clinicians may still be unclear about which doxycycline dose is optimal, and whether the possibility of using SDD40 exists in a wide range of patients regardless of body weight. With some clinicians, consideration of weight-based dosing and progressive severity may blur the options for pharmacologic treatment selection.

In order to investigate whether the patient’s weight or lesion count severity impacts clinical outcomes with using SDD40, the efficacy and safety of SDD40 in treating rosacea were evaluated in randomized controlled trials (RCTs; Phase II, III, and IV) and a subsequent meta-analysis.
Methods
Study design. Three studies are described herein. Study A was a randomized (1:1 ratio), double-blind, placebo-controlled, parallel-group, multicenter, Phase II study in subjects with rosacea to evaluate the safety and efficacy of SDD40 over 16 weeks as an adjunct to metronidazole gel 1% once daily (QD) topically for 12 weeks.7
Study B was a post-hoc analysis of a randomized (1:1 ratio), double-blind, active-controlled (doxycycline 100mg/day), parallel-group, multicenter, Phase IV study in subjects with rosacea over 16 weeks. The purpose of this analysis was to explore whether the number of baseline lesions affected the efficacy of SDD40.18
Study C, the focus of this publication, was a meta-analysis of two randomized (1:1 ratio), double-blind, placebo-controlled, parallel-group, multicenter, Phase III, pivotal studies over 16 weeks. The objective of the meta-analysis was to determine whether the number of baseline lesions affected the efficacy of SDD40.19
Study protocols were approved by independent ethics committees and conformed to the Declaration of Helsinki.
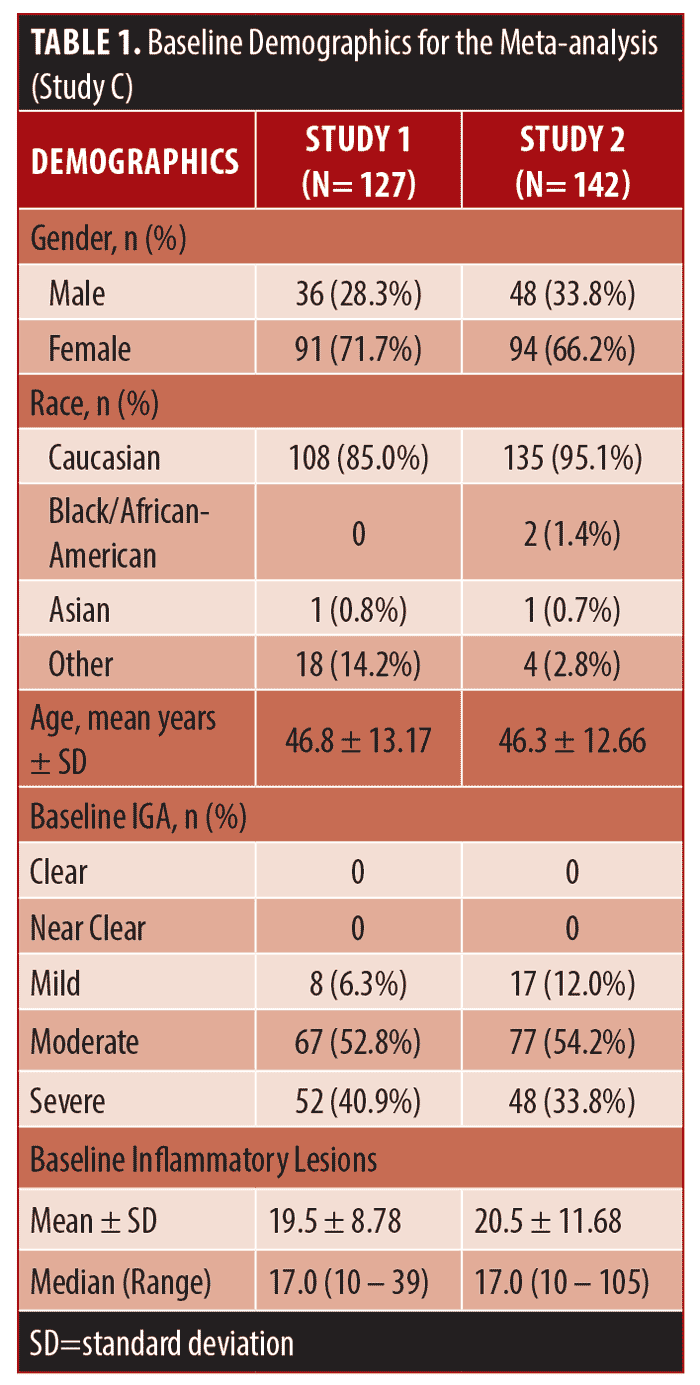
Subjects. For studies A and B, eligible subjects were male and female aged 18 years or older with rosacea, 8 to 40 total lesions (papules and pustules) and no more than two nodules (plus a score of 2 to 5 [mild to very severe] on the Investigator’s Global Assessment [IGA]). For Study C, inclusion criteria were similar although the IGA scale had evolved from a 6-point to a 5-point scale (i.e. removal of the “very severe” category). Both studies of the meta-analysis included male and female subjects aged 18 years or older with mild to severe rosacea (IGA score of 2 to 4), 10 to 40 papules and pustules and no more than two nodules.
Efficacy and safety outcome assessments. For all studies, the primary efficacy endpoint was the change in total inflammatory lesion count (papules, pustules, and nodules) from baseline to Weeks 12 and 16 (only Week 16 for studies B and C). Furthermore, for study C, weights were categorized into underweight (i.e. BMI <18.5), normal (BMI: 18.5-24.9), overweight (BMI: 25.0-29.9), and obese (BMI ≥30.0). Secondary efficacy endpoints included the change in IGA. For studies A and B, adverse events (AEs) were monitored and reported by questioning of the subjects.
Statistical Methods. For all studies, a randomization list was prepared using a computer-generated randomization scheme. Intent-to-Treat (ITT) populations were analyzed, including all randomized patients who took at least one dose of study medication, synonymous with the safety population. For missing data, the last observation (post-randomization) was carried forward and used for the ITT endpoint analysis (last observation carried forward or LOCF approach). For studies A and C, Per-Protocol (PP) populations were also evaluated (a subset of the ITT population without any major protocol violations). Subject disposition, demographics, and baseline characteristics were summarized by descriptive statistics.
An analysis of variance (ANOVA) was performed to compare treatment groups. Moreover, for the meta-analysis (study C), the correlation coefficient was calculated to assess how baseline lesion counts and weight affected the percent change from baseline. A correlation coefficient below 0.75 meant that no linear relationship existed. Also, for studies A and C, IGA scores were analyzed using a Cochran-Mantel-Haenszel (CMH) test to determine the independence or association between variables, stratified by pooled center.
Results
Efficacy. Study A. In this RCT, a total of 72 subjects with papulopustular rosacea were enrolled, and 36 were randomly assigned to each group (metronidazole gel 1% and SDD40 or placebo, respectively). The majority completed the study: 83.3 percent for the SDD40 arm and 94.4 percent for the placebo arm. Demographics were similar between groups, and the majority of subjects were affected by moderate rosacea.
The combination treatment (SDD40 + metronidazole gel 1%) was well tolerated and provided added benefit in reducing inflammatory lesions of rosacea compared with metronidazole gel 1% and placebo. Additionally based on CMH testing, the IGA at each post-baseline visit of the SDD40 group was similar (i.e. not significantly different) compared to baseline. This indicates that SDD40 remained effective regardless of baseline disease severity. Conversely for the placebo group, significant differences were observed for post-baseline IGA scores, meaning that they were dependent on baseline values.
Study B. A total of 91 subjects with papulopustular lesions of rosacea were enrolled, with 44 randomly assigned to the SDD40 group and 47 to the doxycycline 100mg once daily group. The majority of subjects completed the study: 68.2 percent for SDD40 and 78.7 percent for the doxycycline 100mg/day group. Demographics and baseline characteristics were similar between groups. Approximately half of the study population had moderate rosacea, slightly over one-fourth had mild rosacea, and the rest of subjects were affected by severe or very severe rosacea.
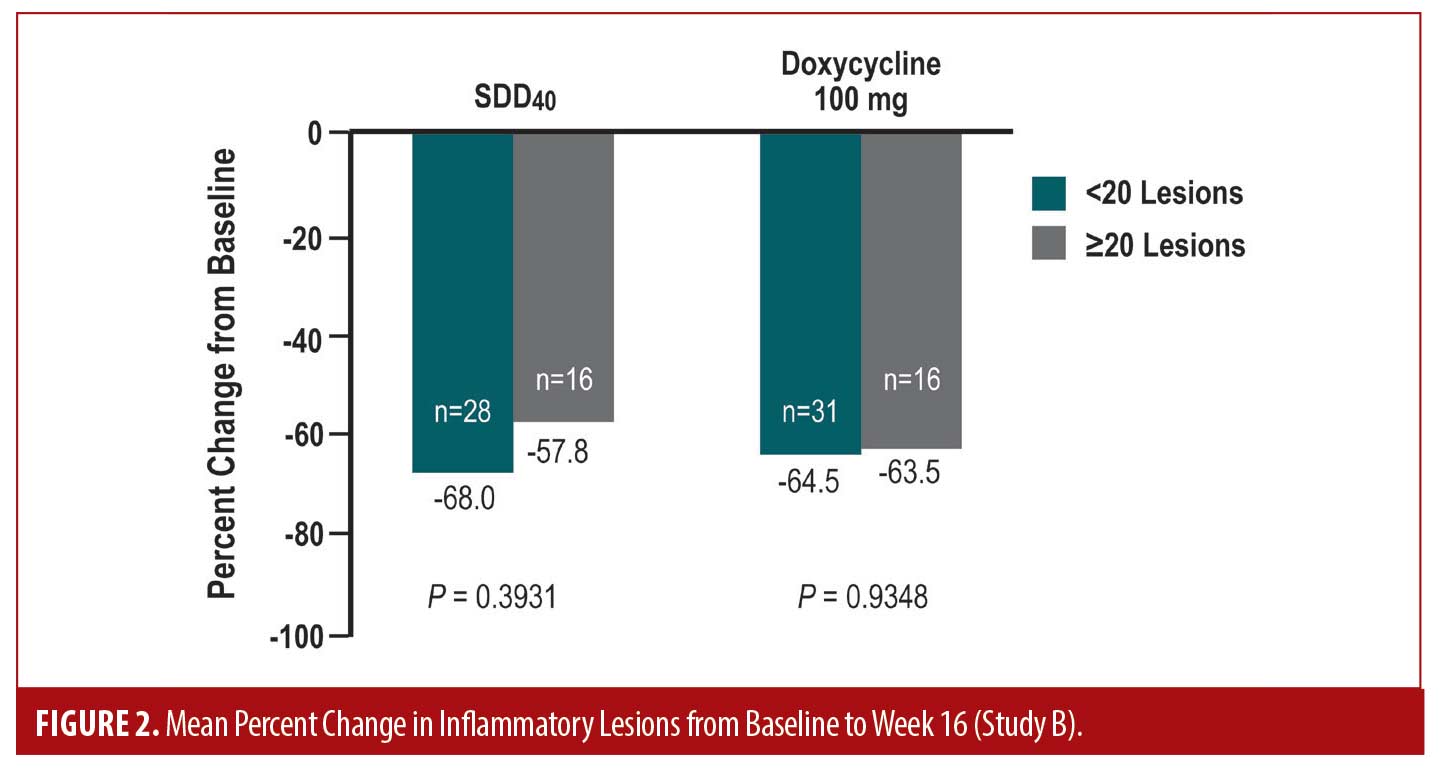
The post-hoc results highlight the efficacy of SDD40, in terms of change from baseline (r= 0.41 at study end) and percent change from baseline in lesion counts (r= 0.25 at study end), independent of baseline lesion counts in either treatment arm. When baseline lesion counts were dichotomized (<20 and ≥20), the mean percent change in lesion counts at any visit appeared to be independent of baseline lesion counts in either treatment arm (Figure 2). The progression of inflammatory lesion count reduction based on evaluation at each time point was nearly identical between both study arms over the 16-week duration of the study.
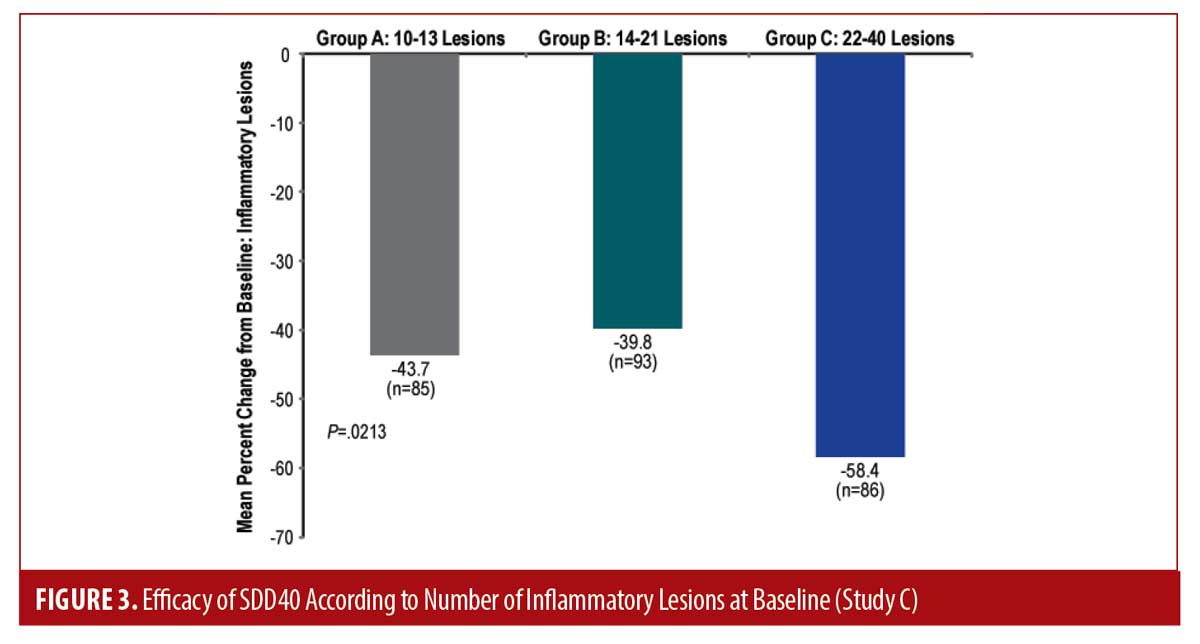
Study C. Results of the meta-analysis were consistent with previously reported results. Subject demographics and baseline characteristics were comparable between studies (Table 1). For both studies (n=127 and n=142), while the efficacy of SDD40 was observed across severities, it was more marked in the severe group of subjects (Figure 3). The mean percent change from baseline was greater in subjects with higher baseline lesion counts (i.e. 22-40 inflammatory lesions) compared to those with fewer lesions (P= 0.0213).
Overall, the efficacy of SDD40 against inflammatory lesions was found to be similar regardless of the number of baseline lesions and subject weight. The correlation coefficient (0.089) from the ANOVA test remained below 0.75, meaning that there was almost no relationship between the number of inflammatory lesions at baseline and the percent change in inflammatory lesions from baseline (Figure 4). The efficacy of SDD40 against inflammatory lesions continued regardless of the number of baseline lesions and weight of the subject (with a correlation coefficient below 0.75), indicating that any potential relationship between weight, baseline lesions, and percent change from baseline in inflammatory lesions was weak (Figure 5). In summary, overweight or obese subjects with severe rosacea cleared at least as well than as those with a normal BMI and milder severity of disease.


Safety. SDD40 showed an overall good safety profile with no to minimal GI-related AEs across studies, and with a lower rate than with doxycycline 100mg/day. Furthermore, there were no reports of vaginal candidiasis or phototoxicity in the Phase III pivotal trials with SDD40, and were rarely or not observed in any other studies. Most AEs were mild or moderate, and no serious AE was related to study drugs. Few subjects discontinued SDD40 due to AEs.
For study A, AEs related to SDD40 were as follows: 1 subject (2.8%) with a probable relationship (stomach discomfort), and five subjects (13.9%) with a possible relationship (sunburn for 2 subjects, nausea, diarrhea, and dermatitis). For study B, treatment-related AEs with SDD40 were less than half that for doxycycline 100mg/day: 5 subjects (11.4%) for SDD40 vs. 13 (27.7%) for doxycycline 100mg/day. AEs with a probable relationship were experienced by three subjects (6.8%) for SDD40 vs. 10 (21.3%) for doxycycline 100mg/day, and a possible relationship by three subjects (6.8%) vs. 3 (6.4%), respectively. At least possibly-related AEs for SDD40 were swollen tongue, tongue disorder, seasonal allergy, pruritus, skin burning sensation, and urticaria (1 subject each), and for doxycycline 100mg/day were nausea (6 subjects), diarrhea, esophageal pain, vomiting (2 subjects each), abdominal pain, upper abdominal pain, dry mouth, dyspepsia, and dysgeusia (i.e. altered taste) (1 subject each).
Discussion
We aimed to investigate whether the patient’s weight or rosacea lesion severity impacts the efficacy of SDD40. Regardless of these parameters, the efficacy of SDD40 was consistent across the evaluated studies and meta-analysis. The treatment was well tolerated, with absent to minimal GI-related AEs across studies.
SDD40 is a well-established treatment of inflammatory lesions of rosacea and can be used among diverse patient populations. This can facilitate treatment selection for clinicians as dose adjustments are not required, the side effect profile is favorable compared to antibiotic-dose doxycycline, and once-daily dosing is convenient for patients. Shared decision-making is important to patients. In line with this, it is important for clinicians to communicate to patients about comprehensive treatment of their rosacea, encompassing skin care, avoidance of triggers, medication use, and general health measures such as nutrition and weight control.20 Indeed, the use of SDD40 can address skin manifestations of rosacea and reduce adverse reactions such as GI side effects. Antibiotic doses of tetracyclines, which are not FDA-approved for rosacea treatment, are associated with a range of other potential side effects. An example is oral minocycline which may be associated with vertigo, hyperpigmentation, and certain rare but significant immunologic reactions.21 Importantly, in a study of rosacea relapse, SDD40 has been shown to be safe and more effective than placebo in reducing flares, when studied up to 12 months.22
The desire to avoid antibiotic activity and to minimize emergence of antibiotic-resistant bacteria when treating rosacea, including sparing of effects on the gut microbiome, support the use of subantibiotic dosing. A recent meta-analysis suggests that obesity has a significantly higher risk (three-fold [odds ratio of 3.41] in Western countries) of small intestine bacterial overgrowth (SIBO).23 Rosacea has also been shown to be associated with several GI disorders,24 further highlighting the need to reduce antibiotic exposure. A 9-month treatment period with SDD40 among patients with periodontitis demonstrated a lack of antibiotic selection pressure and emergence of resistant bacterial organisms that comprise the intestinal or vaginal flora.25 The subantibiotic, anti-inflammatory dose of 40mg (modified release) has efficacy equivalent to the higher dosage of doxycycline (100mg/day), but with fewer side effects.13 Indeed, limiting factors to prescribing antibiotic doses of doxycycline (>50mg/day) include its GI-related side effects (e.g. nausea, vomiting, diarrhea),26 and emergence of antibiotic-resistant bacteria.
Our studies support rosacea treatment using SDD40 as the optimal dosing not only regardless of patient weight, but also independent of rosacea severity. However, results of another recent meta-analysis, albeit of heterogeneous studies of SDD40 in rosacea, suggest a different approach from our findings regarding severity.27 While we agree that initiating SDD40 at an early stage of rosacea development may potentially mitigate the progression of rosacea and reduce associated exacerbations,27 we do not concur that it is mainly suitable for mild cases. Indeed, as shown in our current studies, SDD40 can be used safely and effectively for treatment of papulopustular lesions in all severities of rosacea. Importantly, rosacea treatment should be tailored to address the signs and symptoms of rosacea affecting the individual patient, along with other patient-specific factors. As needed, combination therapies using SDD40 along with other topical agents such as ivermectin, metronidazole, or azelaic acid7-9, 28-29 have also been shown to be effective in the treatment of inflammatory lesions of rosacea.
While some of our observations are limited due to the inherent limitations of retrospective analyses, the consistency between study results including the meta-analysis emphasizes its applicability across a variety of patient types. Future research directions could include a longitudinal study following patients taking SDD40 over several years to expand our knowledge of the course of rosacea over time, patterns of remission, impact on disease progression, and safety data over a very prolonged duration. This could further elucidate long-term SDD40 use for overall rosacea management and evaluate the potential benefits that may occur with reduction of systemic inflammation.
Conclusion
These studies support the use of subantibiotic dose doxycycline as an optimal oral treatment among patients with inflammatory lesions irrespective of their weight or rosacea severity.
Acknowledgments
The authors would like to thank Galadriel Bonnel, PhD, RN, FNP, for editorial assistance.
References
- National Rosacea Society Web site. Information for patients. Accessed 12/6/21 from http://www.rosacea.org/patients/index.php.
- Tan J, Blume-Peytavi U, Ortonne JP, et al. An observational cross-sectional survey of rosacea: clinical associations and progression between subtypes. The British Journal of Dermatology. 2013; 169(3):555–562.
- Rainer BM, Fischer AH, Luz Felipe da Silva D, et al. Rosacea is associated with chronic systemic diseases in a skin severity-dependent manner: results of a case-control study. J Am Acad Dermatol. 2015; 73(4):604–608.
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71(5):973–980.
- Li S, Cho E, Drucker AM, et al. Obesity and risk for incident rosacea in US women. J Am Acad Dermatol. 2017; 77(6):1083–1087.e5.
- Aldrich N, Gerstenblith M, Fu P, et al. Genetic vs Environmental Factors That Correlate With Rosacea: A Cohort-Based Survey of Twins. JAMA Dermatol. 2015; 151(11):1213–1219.
- Fowler JF, Jr. Combined effect of anti-inflammatory dose doxycycline (40-mg doxycycline, usp monohydrate controlled-release capsules) and metronidazole topical gel 1% in the treatment of rosacea. J Drugs Dermatol. 2007; 6(6):641–645.
- Schaller M, Kemény L, Havlickova B, et al. A randomized phase 3b/4 study to evaluate concomitant use of topical ivermectin 1% cream and doxycycline 40-mg modified-release capsules, versus topical ivermectin 1% cream and placebo in the treatment of severe rosacea. J Am Acad Dermatol. 2020; 82(2):336–343.
- van Zuuren EJ, Fedorowicz Z, Carter B, et al. Interventions for rosacea. The Cochrane database of systematic reviews. 2015; 4:CD003262.
- FDA. Oracea capsules [package insert]. Accessed 4/15/22 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/050805s011lbl.pdf
- Di Nardo A, Holmes AD, Muto Y, et al. Improved clinical outcome and biomarkers in adults with papulopustular rosacea treated with doxycycline modified-release capsules in a randomized trial. J Am Acad Dermatol. 2016; 74(6):1086–1092.
- Webster GF. An open-label, community-based, 12-week assessment of the effectiveness and safety of monotherapy with doxycycline 40 mg (30-mg immediate-release and 10-mg delayed-release beads). Cutis. 2010;86(5 Suppl):7–15.
- Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007; 56(5):791–802.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008; 7:573–576.
- Lim HG, Fischer A, Rueda MJ, et al. Prevalence of gastrointestinal comorbidities in rosacea: Comparison of subantimicrobial, modified release doxycycline versus conventional release doxycycline. J Am Acad Dermatol. 2018; 78(2):417–419.
- Theobald K, Bradshaw M, Leyden J. Anti-inflammatory dose doxycycline (40 mg controlled-release) confers maximum anti-inflammatory efficacy in rosacea. Skinmed. 2007; 6(5):221–226.
- Del Rosso JQ. A status report on the use of subantimicrobial-dose doxycycline: a review of the biologic and antimicrobial effects of the tetracyclines. Cutis. 2004; 74(2):118–122.
- Galderma Laboratories, L.P. Clinical Study Report GLI.04.SRE.US10115. A Multicenter, Randomized, Double-Blind, Active-Control Clinical Trial to Determine the Effects of Doxycycline Monohydrate Modified-Release Capsules, 40 mg (COL-101) Administered Once Daily in Conjunction with Metrogel® (metronidazole topical gel, 1%) versus Doxycycline Hyclate Immediate-Release Capsules, 100 mg Administered Once Daily in Conjunction with Metrogel® (metronidazole topical gel, 1%) in Patients with Moderate to Severe Rosacea. Data on file; 2010.
- Preston NJ, Gottschalk RW. Efficacy of doxycycline monohydrate delayed-release capsules, 40 mg is evident regardless of the number of inflammatory lesions at baseline: a meta-analysis from phase 3 studies. Presented at: American Academy of Dermatology Annual Meeting; February 4-8, 2011; New Orleans, Louisiana.
- American Academy of Dermatology Association (AAD). Living with rosacea? How to reduce your risk of other conditions. Accessed 12/6/21 from https://www.aad.org/public/diseases/rosacea/insider/risk-other
- FDA. Solodyn tablets [package insert]. Accessed 12/6/21 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/050808s014lbl.pdf
- Del Rosso JQ, Brantman S, Baldwin H. Long-term inflammatory rosacea management with subantibiotic dose oral doxycycline 40 mg modified-release capsules once daily. Dermatol Ther. 2022;35(1):e15180.
- Wijarnpreecha K, Werlang ME, Watthanasuntorn K, et al. Obesity and Risk of Small Intestine Bacterial Overgrowth: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2020; 65(5):1414–1422.
- Gallo RL, Granstein RD, Kang S, et al. Rosacea comorbidities and future research: The 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018; 78(1):167–170.
- Walker C, Preshaw PM, Novak J, et al. Long-term treatment with sub-antimicrobial dose doxycycline has no antibacterial effect on intestinal flora. J Clin Periodontol. 2005; 32(11):1163–1169.
- FDA. Doryx tablets [package insert]. Accessed 12/6/21 from https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050795s010lbl.pdf
- Husein-ElAhmed H, Steinhoff M. Evaluation of the efficacy of subantimicrobial dose doxycycline in rosacea: a systematic review of clinical trials and meta-analysis. J Dtsch Dermatol Ges. 2021; 19(1):7–17.
- Taieb A, Ortonne JP, Ruzicka T, et al. Superiority of ivermectin 1% cream over metronidazole 0.75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol. 2015; 172(4):1103–1110.
- Husein-ElAhmed H, Steinhoff M. Efficacy of topical ivermectin and impact on quality of life in patients with papulopustular rosacea: A systematic review and meta-analysis. Dermatol Ther. 2020; 33(1):e13203.

