 J Clin Aesthet Dermatol. 2022;15(9):45–49.
J Clin Aesthet Dermatol. 2022;15(9):45–49.
by Anna R. Hurley, MBBS, BSc, MRCS; Joshua P. Totty, MBBS, MRCS, MD, FHEA; and Richard M. Pinder, MBChB, FRCS
Drs. Hurley, Totty, and Pinder are with the Department of Plastic and Reconstructive Surgery at Hull University Teaching Hospitals at Castle Hill Hospital in Cottingham, United Kingdom. Dr. Totty is also with Hull Medical School in Kingston upon Hull, United Kingdom.
ABSTRACT: Background. Nonmelanoma skin cancers (NMSC) have an incidence of 152,000 cases per year in the United Kingdom (UK), which continues to rise. Incomplete excision rates for NMSC are estimated to be around 10 percent and result in patients having a higher risk of recurrence or having to undergo further treatment.
Objective. The objective of our study was to determine whether the use of dermoscopy as an adjunct to clinical examination could improve the rates of incomplete excision in NMSC lesions.
Methods. Electronic literature search of MEDLINE, EMBASE, and Cochrane Central databases plus manual reference checks of articles on dermoscopy use in surgery between inception and November 2020. Two levels of screening were used on 452 studies. A random effects model was used in the meta-analysis, with the DerSimonian-Laird method used to pool data.
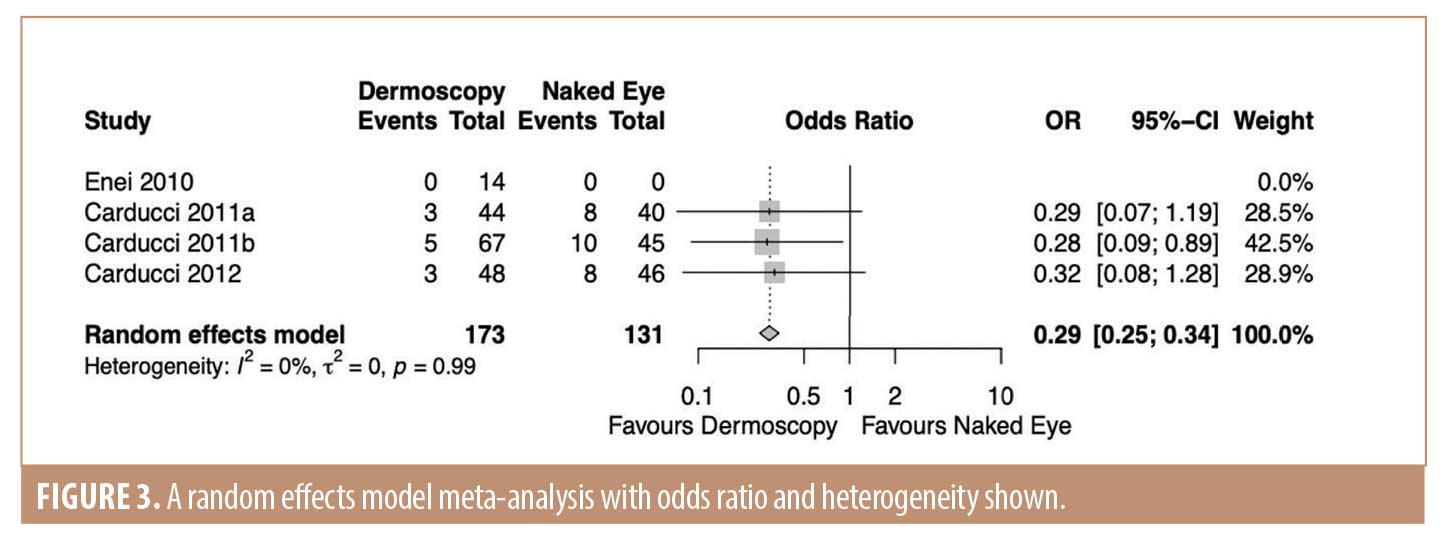
Results. A total of six fully extracted studies were included with a total of 592 patients; with five of these studies reported on basal cell carcinomas and one reported on squamous cell carcinomas. The odds ratio of incomplete excision when guided by dermoscopy was 0.29 (95%CI 0.25; 0.34). Heterogeneity was assessed with the I2 statistic and was found to be 0 percent.
Limitations. The number of studies included was small, with three of the studies from the same authors. Studies included are nonrandomized and as such hold a significant risk of bias.
Conclusion. Incomplete excision rates were reduced when using dermoscopy to mark surgical excision margins in comparison to naked eye evaluation alone.
Keywords. Skin cancer, basal cell carcinoma, squamous cell carcinoma, dermoscopy, non-melanoma skin cancer, surgical excision, margins, positive margins, recurrence
The rates of nonmelanoma skin cancer (NMSC) continue to rise in the United Kingdom (UK), with an incidence of 152,000 new cases each year.1 Surgical excision is the mainstay of curative treatment, but a recent systematic review has highlighted that incomplete excision rates for NMSC is around 10 percent.2
Histological evidence of tumor present at the margin, or inadequately clear of the margin (less than 1mm), leave the patient at higher risk of recurrence without further treatment.3 Methods that can assist in lowering the rate of incomplete excision are, therefore, of great importance to clinicians striving for oncological clearance, as well as healthcare systems in general, as subsequent treatment may be surgery or radiotherapy, which are not without risk, and at extra cost to healthcare systems.
Various methods of margin-control surgery are employed to avoid incomplete excision, particularly for areas of cosmetic concern or where more complex reconstruction may be required, including Mohs micrographic surgery, slow Mohs, and frozen section margin control.4-6 These methods, however, come at great expense to the provider and require specially trained personnel.7 Therefore, a less expensive, more accessible method of potentially reducing the incomplete excision rates would be desirable as long as this is balanced with maintaining effectiveness.
Dermoscopy is the use of magnification to better assess the characteristics and borders of suspicious skin lesions. Its use is promoted by the National Institute of Health and Clinical Excellence (NICE) to support the diagnosis of skin malignancies.8 No such guidance exists on its use as an adjunct to excisional surgery for these lesions. The use of dermoscopy may aid in the identification of characteristics that may not be visible to the naked eye and, as such, may alter the excision margin to include these areas as part of the lesion.9
The aim of this study was to assess the current available evidence regarding the use of dermoscopy as an adjunct to surgical excision of skin lesions.
Methods
Search strategy and selection criteria. This study was prospectively registered on PROSPERO (ID: CRD42021243270) and reported in adherence to Cochrane and PRISMA standards.10,11
All studies reporting outcomes of surgical excision in lesions marked with dermoscopy compared to lesions marked by clinical evaluation alone were considered for inclusion, regardless of setting or publication status. Exclusion criteria consisted of studies that: examined lesions that did not include basal cell carcinoma (BCC) or squamous cell carcinoma (SCC); included Mohs surgery as part of the study method; and reviews. Letters and comments were also excluded.
An electronic search of the literature was conducted using MEDLINE, EMBASE and Cochrane Central encompassing articles published between inception and November 2020. The following search terms were used: “dermoscopy” OR “dermatoscopy” OR “epiluminescence microscopy,” AND “cancer excision” OR “cancer extirpation” OR “cancer resection” OR “neoplastic surgery” OR “tumour excision” OR “tumour exeresis” OR “tumour resection” OR “tumourectomy” OR “skin neoplasms.”
Following the electronic search, bibliographies of included articles were reviewed. Study selection was accomplished through two levels of screening. At Level 1 screening, abstracts were reviewed by two authors (ARH and JPT), with each blinded to the other’s decision to include or exclude, using Rayyan, a web and mobile app for systematic reviews.12 Full articles were then obtained for studies accepted at Level 1, or those where there was conflict over whether the study should be included. Studies with conflict were reviewed by the third author, their decision was discussed with the other reviewers and a final decision about inclusion was made.
Data extraction and quality appraisal.Data was extracted into a bespoke spreadsheet by the principal author of the study. Data on study demographics and design, patient demographics, time period of study, and risk of bias were collected. The primary outcome was the risk of incomplete excision of NMSC.
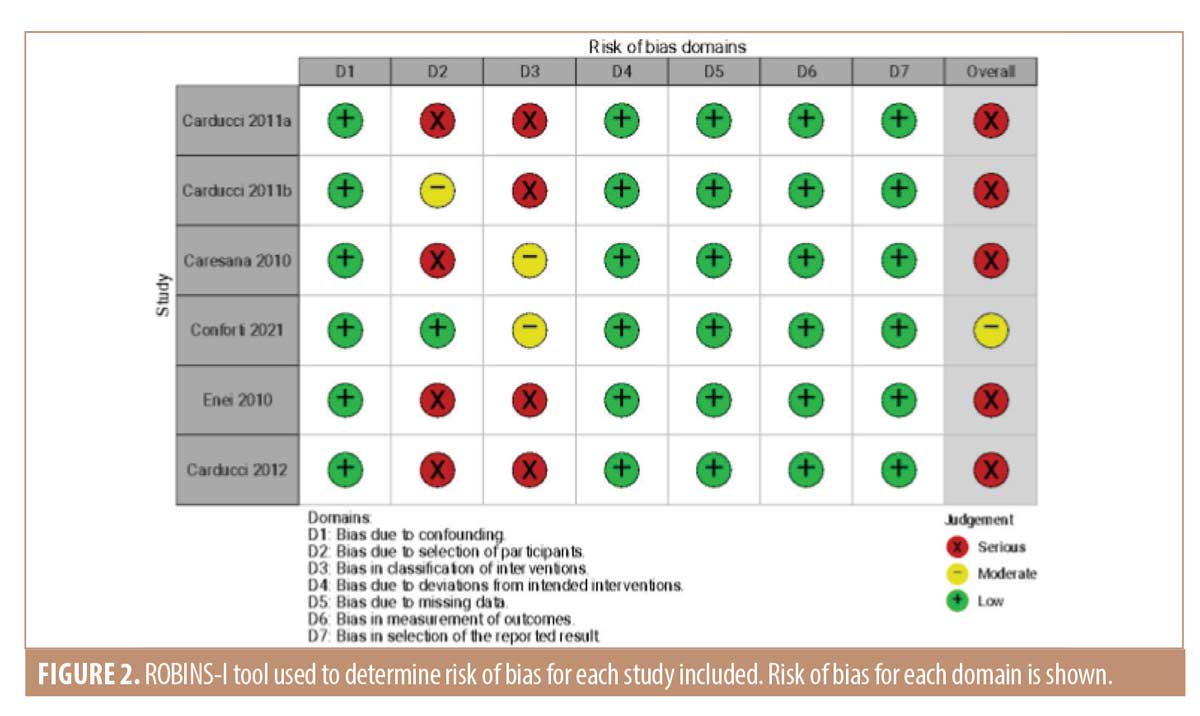
As the studies identified in the literature search were nonrandomized, assessment of risk of bias was performed using ROBINS-I tool (Risk Of Bias In Non-randomized Studies–of Intervention).13 Each domain was given a score of one of the following: “critical risk,” “serious risk,” “moderate risk,” “low risk” or “no information.” The overall risk was determined by the highest level of risk on any domain.
Statistical analysis. Analyses were performed only on the data from the studies included in the data extraction subset. Studies were deemed suitable for synthesis if they reported comparative data pertaining to incomplete excision rates. Data was analyzed using the meta package14 and risk of bias plots produced using the robvis package for R (R Foundation for Statistical Computing, Vienna, Austria).15
Odds ratio of incomplete excision rates were estimated with 95-percent confidence intervals (CI). A random effects model meta-analysis with the DerSimonian-Laird method was used to pool data.16 Statistical heterogeneity was assessed by I2, which corresponds to the proportion of total variation due to between-study heterogeneity. Publication bias and small study effects were not assessed due to there being less than 10 studies included in evidence synthesis.17
Results
Data retrieval. The initial literature review identified 452 citations for screening. Of these, 431 were rejected after reviewing the abstracts. Of the remaining 21 articles, 15 did not meet the inclusion criteria and three studies met criteria only for the catalogue but not further analysis.18-20 Therefore, three studies were available for meta-analysis (Figure 1).21-23 Of the three studies available for analysis, there were six study groups involving 290 participants.
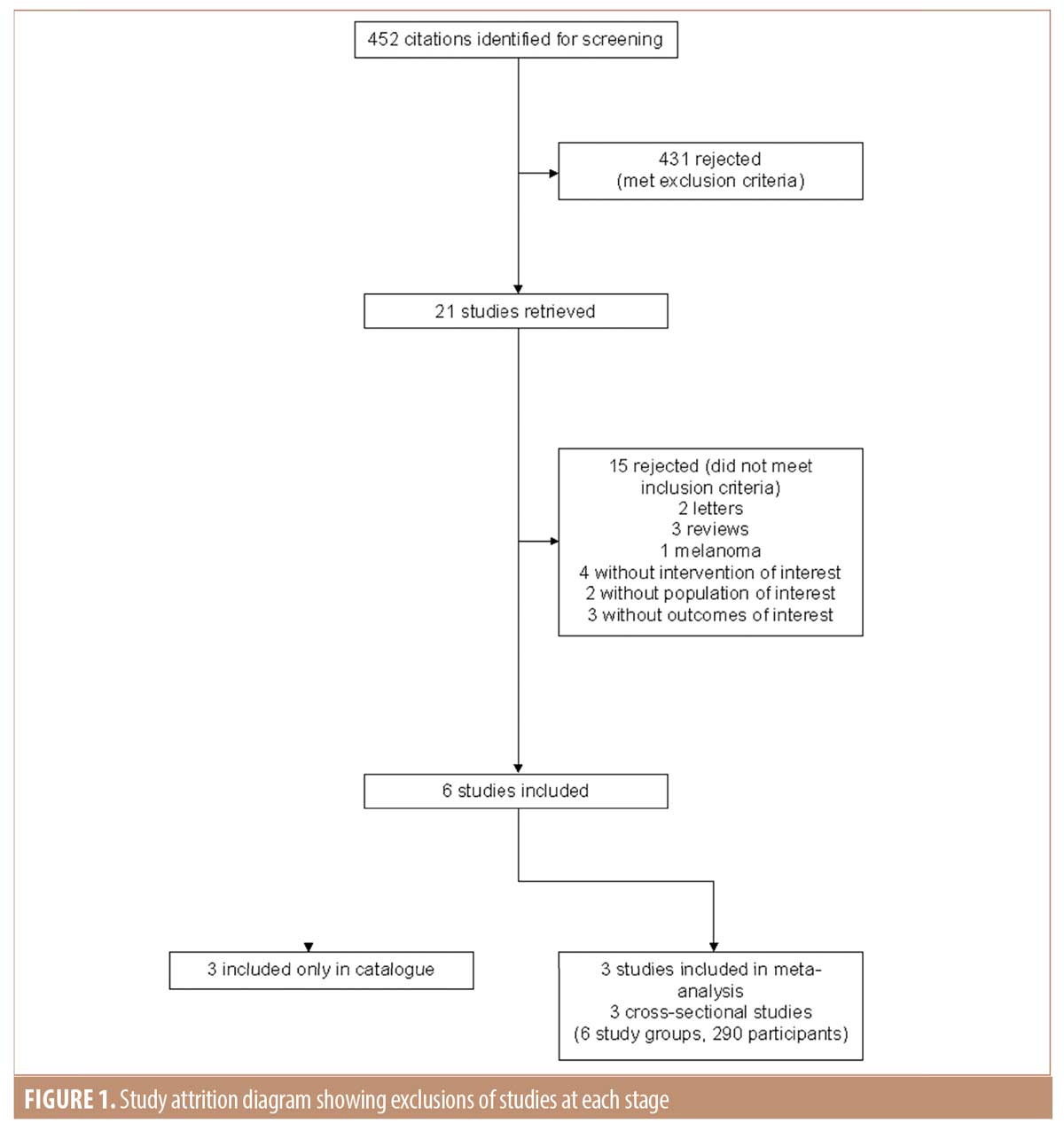
Study characteristics. Characteristics of included studies are outlined in Table 1. The total number of patients across all studies was 592. Of these, 94 had SCC and the remainder had BCC. The presented mean age of study participants was 71.4 (Caresana et al18 did not report on the age of their participants). No study reported a method of randomization for the intervention. All studies reported on the use of dermoscopy as an adjunct to marking of the surgical excision margin. In four out of six of the studies, lesions were of the head and neck.18,19,21,23 For the other two studies, lesions from all areas of the body were included.20,23
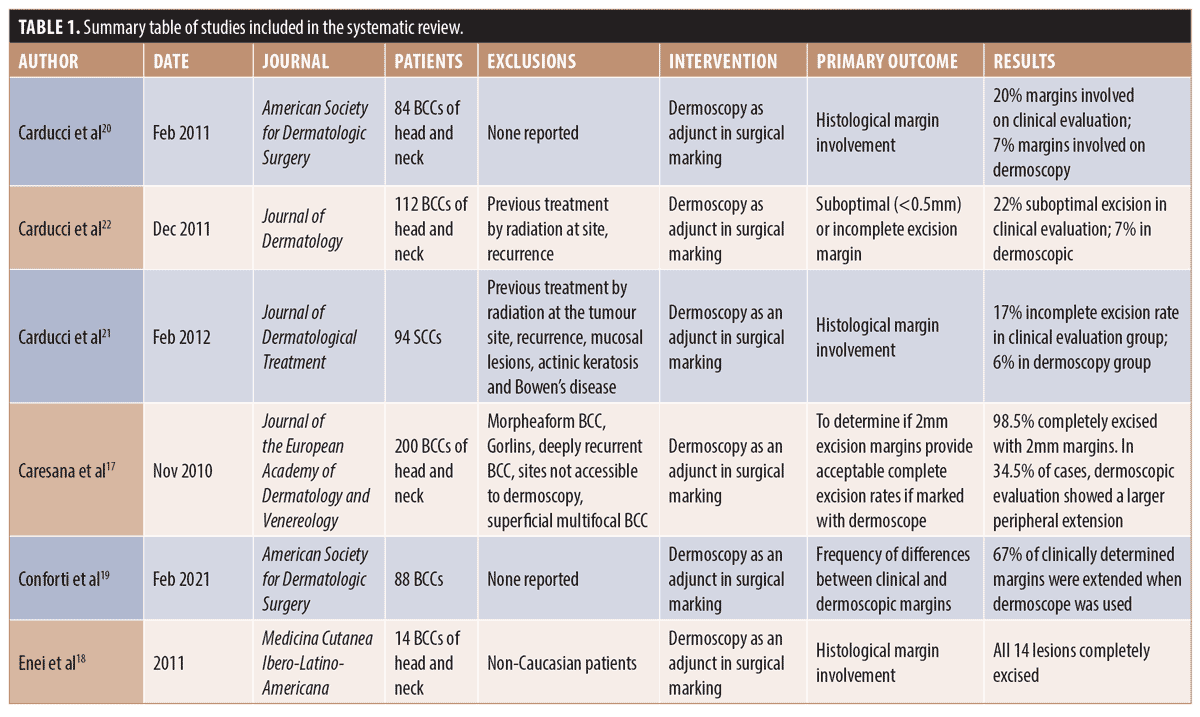
Narrative synthesis. For all studies, dermoscopy was utilized to mark the surgical margin in all participants or in a group selected for intervention. None of the studies reported any adverse effects of using dermoscopy as an adjunct. Three studies by Carducci et al21-23 compared two groups; one group undergoing surgical excision after marking of the margin with naked eye and the other group undergoing surgical excision after marking of the margin with dermoscopy. For two of these studies, BCCs were examined21,23 and for the other study, SCCs were examined.22 A significant reduction in incomplete excision rates when dermoscopy was used in comparison to naked eye was shown in all three studies. Conforti et al20 examined whether using dermoscopy after marking lesions with the naked eye extended the clinical margin. They showed extension of the margins in 67 percent of the lesions. Enei et al19 used dermoscopy to define the surgical margin and reported on incomplete excision rates, but did not include a comparator. In all 14 BCCs examined, the histological margins were satisfactory. Caresana et al18 aimed to determine if 2mm margins were feasible for BCC excision margins if these were marked with the use of dermoscopy as an adjunct. Lesions were marked with the naked eye and subsequently examined with dermoscopy, and extension of the margin recorded. In 69 of 200 cases, the dermoscopic margin was larger than the clinical margin. Only three cases in their cohort were incompletely excised.18
Risk of bias. There was a severe risk of bias identified in the majority of studies (Figure 2). Due to the nature of the intervention (using a dermoscope), blinding was not possible. The three studies by Carducci et al21-23 were nonrandomized comparator control trials comparing the use of dermoscopy against naked eye examination. The other three studies were cross-sectional where dermoscopy was used throughout and histological margin status examined.7-9 One study had a moderate risk of bias due to differing outcome measures.20 Here, the study was not to determine if dermoscopy improved the surgical margin, but rather to decipher any difference between margins marked clinically or dermoscopically; histological margins were not reported.
Data synthesis. Data from three studies were suitable for synthesis.21-23 The odds ratio of incomplete excision when guided by dermoscopy was 0.29 (95% CI 0.25; 0.34) (Figure 3). Heterogeneity was low with an I2 value of 0 percent; all three included studies were produced by the same author. No sensitivity analysis was undertaken due to the small number of studies included in the analysis.
Discussion
Our systematic review and meta-analysis have identified that the use of dermoscopy as an adjunct in marking of margins for excision of NMSC may reduce the risk of incomplete excision. To our knowledge, this is the first systematic review that has focused on the comparison of dermoscopy as an intervention in marking of surgical margins to determine the primary outcome of incomplete excision rate.
NMSCs are most commonly found on the head and neck due to the exposure to ultraviolet light. Incomplete excision rates have been reported to be as high as 10 percent, and even up to 17 percent for periorbital lesions.2,24 Incomplete excision carries with it a risk of recurrence, in the case of SCCs metastasis, and almost always requires further treatment. In cosmetically sensitive areas, the aesthetic outcome sometimes overshadows the aim of oncological clearance and so as little tissue is removed as possible to minimize the defect size through compromising on the oncological margin. Excision of the cancer must be the primary aim, and margin control surgery is often rightly considered for primary excision, and in cases of incomplete excision. In the National Health Service in the UK, this often requires the patient to visit a specialist center, and also comes at an expense to the health system due to the expertise and equipment required.25 Four of six of the included studies involved lesions of the head and neck and all had significant improvements in incomplete excision rates when using dermoscopy to mark the margin.18,19,21,23 While not a substitute for margin control surgery, dermoscopy is a simple adjunct that may help prevent the need for further intervention.
Dermoscopy has been widely shown to be a valuable tool in improving the accuracy of diagnosis of NMSC when compared to the naked eye.26,27 It is a noninvasive technique commonplace in dermatology clinics. The National Institute for Health and Care Excellence guidelines in the UK recommend the use of dermoscopy for diagnosis of lesions reflecting the benefits of use in diagnosis but do not highlight its potential in the operating theater.8 However, I.C. guidelines from the British Association of Dermatologists (BAD) recommend that peripheral tumor margins should be determined under bright lighting as well as magnification or dermoscopy when excising SCC.28 There is no mention of magnification or dermoscopy for marking of margins in the management of BCC, which may reflect the lack of evidence in the area and why further work is of importance. If the use of dermoscopy for tumor margins is being recommended for SCC it would seem appropriate therefore that its use would be of benefit for all NMSC excisions.
Although the number of studies in this field are limited, this systematic review provides new evidence of the use of dermoscopy to improve the accuracy of surgical margin determination and reason to extrapolate the BAD guidelines of the use of dermoscopy for margin determination for all NMSC.
It is evident that dermoscopy has its benefits in diagnosis, and those who are routinely examining patients with skin lesions are recommended to have training in its use.8 This training, however, is limited in specialities outside of dermatology. Other specialities such as plastic surgery may be involved with excising these lesions and hence require dermoscopy skills for diagnosis. A recent correspondence piece highlighted the need for more training in dermoscopy for the plastic surgeon in an effort to improve the efficiency of skin cancer services; it also reported that clinicians who regularly used dermoscopy listed significantly fewer patients for surgery.29 This suggests that improving training may also be of benefit in reducing the number of benign lesions excised and hence patients being put at risk of surgical harm for an unnecessary excision. Even short training sessions have been shown to be of benefit in clinical diagnosis, with one study showing a one-hour course improved the confidence of a benign diagnosis significantly.30
Although this review highlights a benefit of the use of dermoscopy in the operating theater to reduce incomplete excisions, increased access to training is required to ensure true benefit with a clinician that is confident in its use.
Limitations. This review is not without limitations. Firstly, the number of studies included is small, reflecting a paucity of available evidence. Three of the four studies included in the meta-analysis were from the same authors and therefore likely to represent a similar patient group, with study designs likely to encounter similar bias.21-23 It may be that two of their studies included the same patient population, which further decreases the study population in the review. Furthermore, the studies included in our review were nonrandomized and as such hold a significant risk of bias. No clear method of randomization was reported in studies with two arms.21-23 Studies are also hampered by a lack of blinding of the intervention, as it is not possible to blind the surgeon to the use of dermoscopy. However, some studies have attempted to overcome this by taking dermoscopic photographs of the lesions in order to independently verify findings.19-23
In the operating theater, marking of the surgical margin is often performed while wearing surgical loupes under operating lights. Magnification of loupes varies from around 2.5 to 3.5x. None of the studies in this review included this as part of their methodology, and therefore it would seem that marking has been done with the naked eye. For a true comparison of whether dermoscopy has a benefit over clinical evaluation, a robust, prospective, randomized, controlled trial is needed.
Conclusion
This study examines a relatively inexpensive and readily available method of improving incomplete excision rates which most plastic surgeons and dermatological surgeons will be trained in using for skin cancer clinics and therefore be familiar with. The use of dermoscopy as an adjunct to surgical margin marking may provide the surgeon with a quick and accurate method that can easily be introduced in operating theaters and can improve the patient experience by reducing the risk of an involved histological margin in NMSC. However, the quality of the available evidence is low and at significant risk of bias, and therefore further research is required in this area.
We would recommend that dermatoscopes be routinely available and used in operating departments; that training in their use be provided for surgeons; and that measurement of excision margins, along with the use or otherwise of dermoscopy, be recorded to aid future analysis of the utility of dermoscopy.
Acknowledgments
The authors would like to thank Tim Staniland of Hull University Teaching Hospitals NHS Trust Library for his assistance with the literature review for the paper.
References
- Cancer Research, Non-melanoma skin cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/non-melanoma-skin-cancer#heading-Zero.
- Nolan GS, Kiely AL, Totty JP, et al. Incomplete surgical excision of keratinocyte skin cancers: a systematic review and meta-analysis. Br J Dermatol. 2021;184(6):1033–1044.
- Ranjan R, Singh L, Arava SK, et al. Margins in skin excision biopsies: principles and guidelines. Indian J Dermatol. 2014;59(6):567–570.
- NWJ Smeets. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004 Nov;364(9447):1766–1772.
- Nicoletti G, Brenta F, Malovini A, et al. Study to determine whether intraoperative frozen section biopsy improves surgical treatment of non-melanoma skin cancer. Mol Clin Oncol. 2013 Mar;1(2):390-394.
- Ghauri RR, Gunter A, Weber RA, et al. Frozen section analysis in the management of skin cancers. Ann. Plast. Surg. 1999 Aug;43(2):156-60.
- Raviskly L, Brodland DG, Zitelli JA, et al. Cost Analysis: Mohs Micrographic Surgery. Dermatologic Surg. 2012 Apr;38(4):585-94.
- National Institute for Health and Care Excellence, “Skin cancer,” Skin cancer quality standard, 2016. https://www.nice.org.uk/guidance/qs130/resources/skin-cancer-pdf-75545412324037.
- Lallas A. Update on non-melanoma skin cancer and the value of dermoscopy in its diagnosis and treatment monitoring. Expert Rev. Anticancer Ther. 2013 May;13(5):541-58.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
- Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372.
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
- Sterne J, Hernán M, McAleenan A, et al. Assessing risk of bias in a non-randomized study, in Cochrane Handbook for Systematic Reviews of Interventions. Higgins J, Thomas J, Chandler, Cumpston M, Li T, Page M, and Welch V, Eds. 2021.
- Schwarzer G. General Package for Meta-Analysis. 2021. Available at: https://cran.r-project.org/web/packages/meta/meta.pdf.
- McGuinness L. robvis: An R package and web application for visualising risk-of-bias assessments. 2021 Jan;12(1):55-61.
- DerSimonian, R. and Laird, N. Meta-Analysis in Clinical Trials. Controlled Clinical Trials. 1986;7: 177–188.
- Sterne JAC. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011 Jul 22;343:d4002.
- Caresana G and Giardini R. Dermoscopy-guided surgery in basal cell carcinoma. J Eur Acad. Dermatol Venereol. 2010 Dec;24(12):1395-1399.
- Enei M, Paschoal F, Valdes R, et al. A dermoscopic contribution to the establishment of minimal surgical margins of resection in basal cell carcinoma of the face and scalp. Med Cutan Iber Lat Am. 2011;39(4).
- Conforti. C Dermoscopic Findings in the Presurgical Evaluation of Basal Cell Carcinoma. A Prospective Study. Dermatologic Surg. 2021 Feb 1;47(2):e37-e41.
- Carducci M, Bozzetti M, Foscolo AM, et al. Margin detection using digital dermatoscopy improves the performance of traditional surgical excision of basal cell carcinomas of the head and neck. Dermatologic Surg. 2011 Feb;37(2):280–285.
- Carducci M, Bozzetti M, De Marco G, et al. Preoperative margin detection by digital dermoscopy in the traditional surgical excision of cutaneous squamous cell carcinomas. J Dermatolog Treat. 2013 Jun;24(3):221–226.
- Carducci M, Bozzetti M, De Marco G, et al. Usefulness of margin detection by digital dermoscopy in the traditional surgical excision of basal cell carcinomas of the head and neck including infiltrative/morpheaform type. J Dermatol. 2012 Apr;39(4):326-30.
- Griffiths RW. Audit of histologically incompletely excised basal cell carcinomas: recommendations for management by re-excision. Br J Plast Surg. 1999 Jan;52(1):24-8.
- British Association of Dermatology. Service guidance and standards for Mohs micrographic surgery. 2021. https://www.bad.org.uk/shared/get-file.ashx?itemtype=document&id=6346. 2021 Apr 16.
- Reiter O. The diagnostic accuracy of dermoscopy for basal cell carcinoma: A systematic review and meta-analysis. J Am Acad Dermatol. 2019 May;80(5):1380-1388.
- Fargnoli MC, Kostaki D, Piccioni A, et al. Dermoscopy in the diagnosis and management of non-melanoma skin cancers. Eur J Dermatol. 2012 Jul-Aug;22(4):456-63.
- Keohane SG. British Association of Dermatologists guidelines for the management of people with cutaneous squamous cell carcinoma 2020. Br J Dermatol. 2021 Mar;184(3):401-414.
- Brennan MC, Kabuli MN, Dargan MD, et al. A Short Correspondence Piece to the Editor in Chief: The need for increased training in the technique of dermoscopy amongst plastic surgeons and the under recognised value of dermoscopy in the assessment of non-pigmented cutaneous lesions. J Plast Reconstr Aesthetic Surg. 2022 Jan;75(1):496-498.
- Benvenuto-Andrade C. Level of Confidence in Diagnosis: Clinical Examination Versus Dermoscopy Examination. Dermatologic Surg. 2006 May;32(5):738-44.

