 J Clin Aesthet Dermatol. 2023;16(7):45–53.
J Clin Aesthet Dermatol. 2023;16(7):45–53.
by Lisa F. Fronek, DO; T. Alex Harrison, DO; Taylor E. Gray, DO; and Travis W. Blalock, MD
Dr. Fronek is with Bighorn Mohs Surgery and Dermatology at Scripps Clinic in La Jolla, California. Dr. Harrison is with HCA Healthcare/USF Morsani College of Medicine at the Largo Medical Center, in Largo, Florida. Dr. Gray is with the Department of Dermatology at the Ohio State University in Columbus, Ohio. Dr. Blalock is with the Department of Dermatologic Surgery at Emory University School of Medicine in Atlanta, Georgia.
FUNDING: No funding was provided for this article.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
Removal of cutaneous malignancies on the hand and fingers can result in challenging surgical defects to close. The dermatologic surgeon must not only be highly skilled, but also be knowledgeable regarding the complex anatomy of this area to perform reconstruction that provides optimal functional and cosmetic results. This review highlights key anatomic factors that must be considered when operating in this region. Wound management options discussed below include secondary intention, primary linear repair, local skin flaps, interpolation flaps, and skin grafting. The surgeon’s choice is based on defect size, the presence/absence of adjacent skin laxity, and other patient-specific factors that may impact healing such as medical comorbidities, utilization of anticoagulant medications, and smoking status. This manuscript serves as an up-to-date review of closure considerations and techniques for physicians who surgically treat cutaneous malignancies of the hand and fingers.
Keywords: dermatologic surgery, squamous cell carcinoma, basal cell carcinoma
Treatment of cutaneous malignancies on the hand presents a challenge due to unique anatomic and functional considerations. Squamous cell carcinoma (SCC) represents the majority of cutaneous malignancies of the hand, followed by basal cell carcinoma (BCC) and malignant melanoma (MM).1,2 Surgical excision, notably Mohs micrographic surgery (MMS), remains the treatment of choice for SCC and BCC on the hand.3,4 Use of MMS for MM is increasing due to the benefits of complete margin assessment and preservation of normal tissue.4,5,6 This review will discuss pertinent anatomy of the dorsal hand that dermatologic surgeons must consider, excluding the wrist and nail unit. We will also present reconstructive options and considerations when faced with surgical defects on the dorsal hand.
Anatomical Considerations
A thorough understanding of relevant anatomy is critical in designing functional and cosmetically optimal repairs while avoiding surgical complications. Surface anatomy of the hands and digits can be divided into palmar and dorsal sides, or radial and ulnar sides. The thumb is divided into subsections based on the carpometacarpal (CP) joint, metacarpophalangeal (MCP) joint, and interphalangeal (IP) joint. Finally, the remaining four digits of each hand are divided into subsections based on the CP joint, MCP joint, proximal interphalangeal (PIP) joint, and distal interphalangeal (DIP) joint. The corresponding anatomical creases are named according to the underlying joint space.
Differences in physiologic function, histology, and topography exist between the dorsal and ventral hand. The skin overlying the dorsal hand is thin and elastic, with a paucity of subcutaneous fat that allows space for soft tissue swelling following surgery. The extensor tendons lie deep to dorsal skin and subcutaneous tissue. The dorsal veins and lymphatics lie superficial to these tendons within the subcutaneous tissue, and travel between each MCP joint proximally toward the wrist. The thenar and hypothenar eminences and corresponding fat pads are topographic landmarks. Each digit contains fat pads that arise between each digital flexion crease. Because there is minimal movement within these flexion creases, they are suboptimal for the placement of surgical incisions [7]. Langer’s lines, representing skin tension lines, occur within the horizontal plane on the dorsum and generally follow skin markings on the palmar surface. The primary movements of the hand (flexion and extension) occur perpendicular to Langer’s lines; thus, surgical incisions should be planned parallel to Langer’s lines.7,8
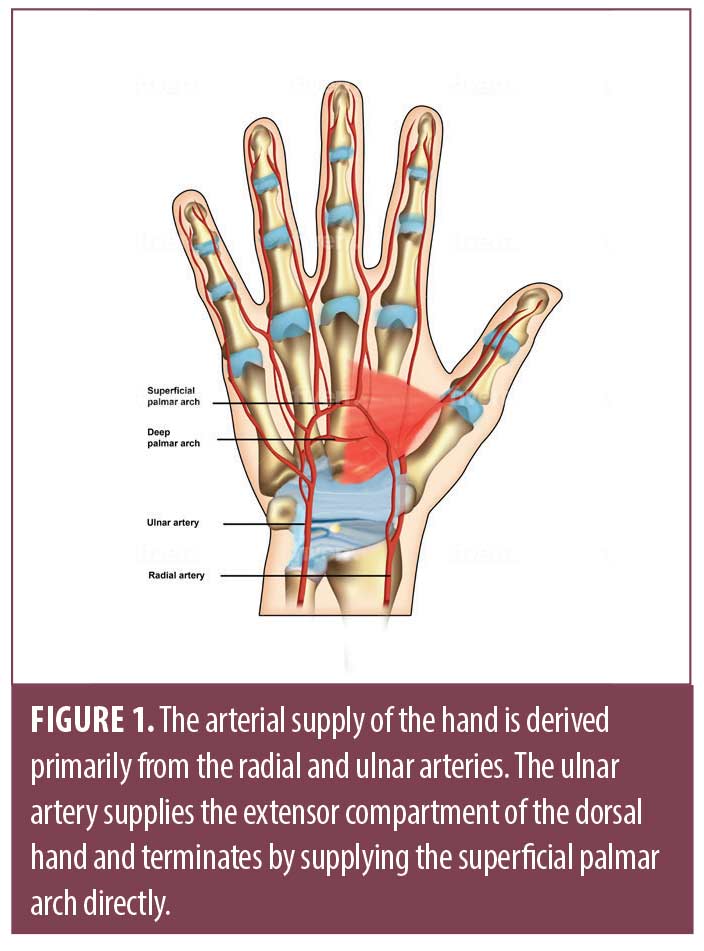
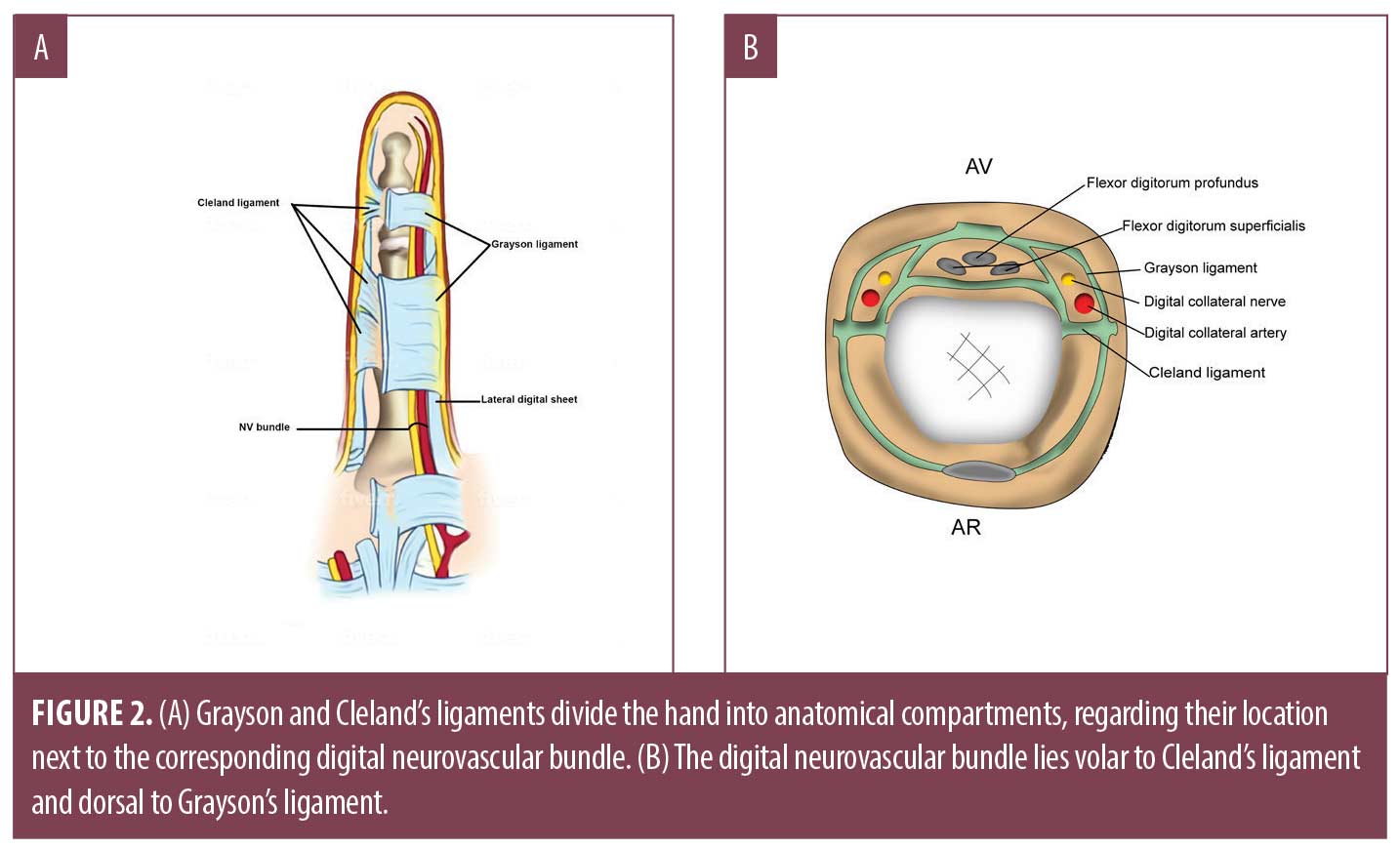
The arterial supply of the hand is derived from the radial, ulnar and occasionally, median arteries. The ulnar artery supplies the extensor compartment of the dorsal hand and then travels along the medial aspect of the distal forearm through Guyon’s canal.7,8 The terminal branch of the ulnar artery supplies the superficial palmar arch directly (Figure 1). Individual digital arteries are derived from this superficial palmar branch, which distribute tributaries at the web spaces of each digit. While in the palm, these arteries remain volar to the corresponding digital nerves. When they traverse into the digit, they lie posterior to the digital nerves. There are two notable ligaments that separate the hand into anatomical compartments based on their relationship with the neurovascular bundle.9 The neurovascular bundle within each digit lies volar to Cleland’s ligament, yet dorsal to Grayson’s ligament (Figures 2 and 3).7,8 The radial artery primarily supplies the deep palmar arch, the thumb, radial side of index finger, and finally, the middle and ring finger. The superficial and deep veins within the hand form corresponding arches, which empty into three dorsal metacarpal veins; these combine and contribute to the prominent cephalic and basilic veins within the forearm. Lymphatic ducts follow venous drainage and nodes may be palpable in the cubital fossae, antecubital fossae, and the axillae.
Sensory innervation of the hand is provided by the radial, median and ulnar nerves. The ulnar nerve supplies the medial third of the volar hand, volar aspect of the fourth and fifth digits, and dorsal aspect of fourth and fifth digits. The median nerve provides sensation to the medial aspect of the volar thumb, the lateral aspect of the volar palm and digits, and the dorsal aspect of the index finger and middle finger. Finally, the radial nerve provides sensation to the lateral aspect of the volar thumb, the dorsal aspect of the thumb and lateral aspect of the dorsal hand.
Knowing the sensory innervation of the hand is paramount when performing peripheral nerve blocks. Nerve blocks require a smaller volume of local anesthetic, decreasing the risk of systemic toxicity and tissue distortion caused by local infiltration.10 To perform a median nerve block, the surgeon identifies the palmaris longus and flexor carpi radialis tendons at the level of the proximal wrist crease; the injection is placed radial to the palmaris longus tendon. For an ulnar nerve block, the surgeon identifies the flexor carpi ulnaris tendon at the proximal wrist crease, injecting just radial to this tendon. Two injections are placed to anesthetize the radial nerve. The surgeon identifies the radial artery and radial styloid bone at the level of the dorsal wrist. The first injection is placed lateral to the radial artery at the level of the radial styloid bone; the second occurs at the dorsal midline of the wrist.10 Many experts recommend aspirating before each injection to decrease risk of arterial injection. While there is conflicting information about maximum amount of anesthesia to be used, Kachare et al11 advocate for a maximum of 5mL.
Wound Management Options
Secondary intention. Secondary intention (SI), the process by which a surgical defect is left to heal on its own, while often used for concave anatomic areas, may be an excellent choice for select dorsal hand defects. Preserved adnexal structures of superficial surgical defects provide a source of keratinocytes used for re-epithelialization.12 However, extended healing time or poor cosmetic outcome may result if utilized for defects that involve subcutaneous fat, tendon or muscle.12 Importantly, exposed tendons are at risk of desiccation and degradation, leading to joint contraction and temporary or permanent disability, if not covered with a local skin flap or graft.13,14 While Snow et al. demonstrated adequate healing with low infection rates following SI in 91 patients with exposed bone defects on the face and scalp, this topic remains controversial and should be utilized with caution in highly mobile areas such as the hand.13–18 Surgeons may choose to avoid SI when defects reach a certain diameter. However, in a retrospective study of post-MMS wounds on the hands/fingers performed by Bosley et al,16 defect size was not a significant factor in healing time. Specifically, the authors documented SI healing with excellent functional and cosmetic results in patients with surgical defects up to 6.0cm on the dorsal hand and 2.0cm on the dorsal aspect of the finger.16
Comparatively, SI is cost effective, technically simple, associated with reduced incidence of postoperative complications, specifically hematoma and dehiscence, and results in lower rates of postoperative bleeding and pain.16,18,19 Additionally, many studies demonstrate lower rates of surgical site infection (SSI) following SI, with few groups arguing the contrary.15,16,18–20 Potential for scar contraction is necessary to consider after dermatologic surgery on the hand. In a retrospective review of thirty-seven patients who underwent SI after MMS on the dorsal hand, Bosley et al16 demonstrated the efficacy and positive functional and cosmetic results when utilizing SI on the dorsal hand. Corroborating this finding, in a prospective case series examining SI on the dorsal hand, Lateo et al. found that zero patients experienced mobility sequelae.18
Healing time after SI is determined by area, depth, surrounding tissue allowing for contraction, and the development of over-granulation tissue.18,19 The minimal subcutaneous fat on the dorsal hands allows for rapid granulation of tissue, making SI a viable repair option on the dorsal hand.18,19 Skin quality should also be considered, notably in elderly patients with fragile skin.18 Atrophic skin due to chronic ultraviolet light exposure makes surgical repair difficult with an atrophic dermis; thus, SI avoids skin tearing during repair.18 Certain patients prefer SI due to reduced operative time and decreased restrictions on postoperative activity.21 The most significant disadvantage of SI is delayed healing, which may be exacerbated by co-morbidities like nicotine dependence or peripheral arterial disease.12,18,21,22 Shape of scar may also be discussed with the patient, as some may prefer a linear versus a stellate or circular-shaped scar.
Primary repairs. Primary linear repair (PLR) is one of the first considerations in the dermatologic surgery reconstructive ladder.23 Advantages of PLR include design simplicity, versatility, operative time, and cost-effectiveness.23 Limiting factors may include defect size and the relative tension of the skin overlying the dorsal hand.24 Because the primary movements of the hand (flexion and extension) occur perpendicular to Langer’s lines, one should plan incision lines with this in mind.7 In patients who lack skin laxity of the dorsal hand, a primary linear repair may lead to a standing cone defect, increased wound tension, and possible dehiscence.21 Notably, the hand may allow for spontaneous regression of standing cones less than 8mm in height.25 Additionally, if particularly taut following a PLR, increased postoperative pain and significant limitation of finger, hand, or wrist movement may result in the postoperative period.21 There are no studies to date analyzing functional outcome, cosmetic outcome, or rates of SSI following PLR on the dorsal hand following MMS.
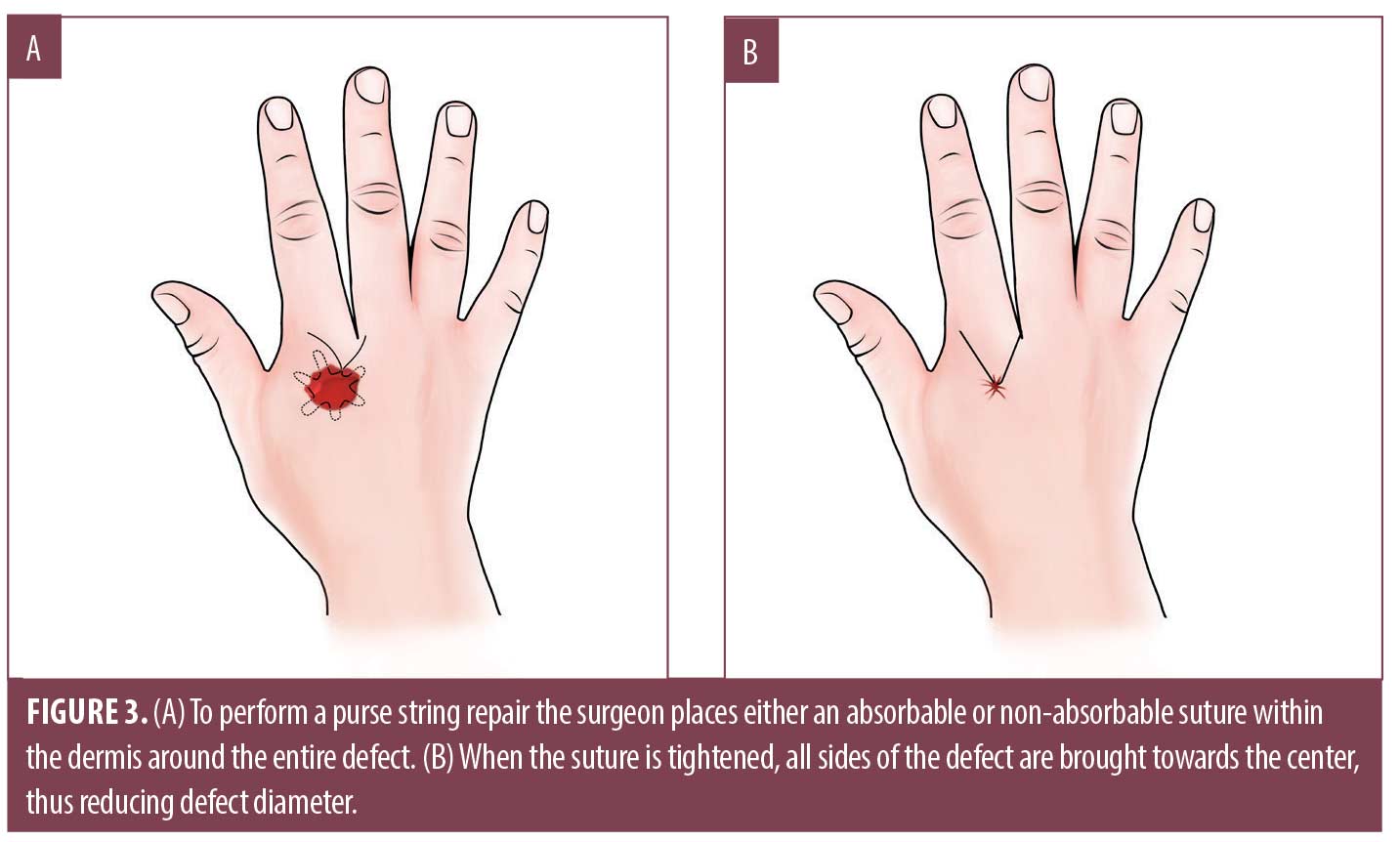
The purse string repair is used for complete or partial closure of round defects.25 It is accomplished by placing either an absorbable or non-absorbable suture horizontally in the dermis or percutaneously around the entire perimeter of the wound (Figure 3A).16 When tying the suture, all sides of the defect are brought towards the center, reducing the wound diameter (Figure 3B).26,27 This is particularly helpful when significant laxity exists in several directions around the defect.16,27 Advocates maintain that the purse string repair accelerates healing time by reducing overall defect size, while generally retaining good functional and cosmetic results.16,26,28 It is theorized that reduced defect size results in decreased postoperative pain, reduced risk for dehiscence, and reduced SSI.26 The purse string repair may also be used as a complementary procedure with local skin flaps, full thickness skin graft (FTSG) or split thickness skin graft (STSG).28 Disadvantages of the purse string repair include risk of tearing atrophic skin and skin pleat formation; however, many studies have demonstrated adequate cosmetic outcomes.16
Allografts, skin substitutes, and xenografts. Skin grafts represent an important consideration for repair on the dorsal hand. They can be utilized when a defect is too large to be closed primarily or when a flap cannot be used due to unsatisfactory local skin reservoir or risk of functional impairment. STSGs use epidermis and upper papillary dermis, while FTSGs are comprised of epidermis and full thickness dermis.13,29 STSGs can be obtained using a safety razor blade, Weck blade, Paget dermatome, or scalpel; the donor site is often allowed to heal by SI, simplifying post-operative care.13 As STSGs are thinner, they have a low metabolic demand and lower failure rates.13,29,30 Furthermore, they allow for easier detection of skin cancer recurrence. Disadvantages include decreased quality of sensation, poor cosmesis, and increased risk of developing contracture in comparison to FTSGs.13,30 An adequate donor site for utilization of a FTSG is similar in color, texture, and thickness to the recipient site. Common donor areas considered for defects of the dorsal hand include the medial brachium, antecubital fossa, volar wrist, and inguinal fold.30 Burow’s grafts serve as an excellent skin match if the surrounding actinic damage is minimal. Slightly oversizing the graft can help reduce the risk of contraction and functional impairment of the dorsal hand. In a review of 21 defects that underwent FTSG on the dorsal hand following MMS, 100 percent of patients reported excellent functional ability.30
Regardless of the type of graft being utilized, a few considerations remain the same. Appropriate size should be estimated with the patient’s hand in a fist position, which maximizes the graft area and minimizes post-operative wound tension.13 Bolster dressings and pressure dressings should be applied and left in place until suture removal to reduce post-operative hematoma and seroma.13 Limiting hand mobility with hand splints or wrist braces is important to ensure graft survival. Although a topic of debate, both STSGs and FTSGs may have increased risk of failure when placed over exposed bone, tendon, or cartilage. Therefore, it is recommended to avoid utilizing grafts over defects without viable peritenon, periosteum, or perichondrium.29,30
Surgeons may also opt for skin alternatives composed of human, non-human (xenograft), or partially synthetic tissue. The use of dehydrated human amnion/chorion membrane (dHACM) has increased in recent years, as it expedites wound healing via production of growth factors.31,32 While originally designed for non-healing wounds caused by diabetes or vascular insufficiency, dHACM allografts have demonstrated efficacy in large surgical defects unamenable to closure following dermatologic surgery.33 Regarding uses of xenografts, Yang et al. followed 220 patients who had an acellular porcine xenograft placed following MMS. The authors ultimately advocate for the use of porcine xenograft to augment SI, as it provides barrier protection, pain reduction, and limits tissue desiccation.34
Dermal substitutes followed by delayed skin grafting or combination closures are additional means for repair, especially for larger defects with exposed tendons on the dorsal hand. Second intention, local flaps (advancement or rotation), and skin grafting may result in tendon desiccation, excess tension, prolonged immobilization, and decreased range of motion from contraction.14 A combination of closures, for example, a transposition flap followed by FTSG, may be optimal in certain situations. Etzkorn et el., described a case in which a single lobed transposition flap from the dorsal wrist was used to repair a large primary defect on the dorsal hand.14 A FTSG from the abdomen was then used to repair the secondary defect on the dorsal medial wrist. In these instances, preserving function of the dorsal hand is of utmost importance.
Local skin flaps. Local skin flaps are indicated in defects deemed unfit for SI, or when PLR would compromise function or result in excessive tension. The local flaps discussed below include advancement, rotation, and transposition flaps. These surgical repairs are appropriate when there is exposed tendon, bone, or cartilage to ensure a reliable and adequate blood supply, and to prevent tissue desiccation, which may impact function or cause local tissue necrosis.35 Due to their proximity to the defect, local flaps are advantageous in matching tissue color and texture.21,36 When well executed, they result in minimal tissue contraction and can be performed as a single stage procedure.21,35 Clinicians should perform a finger pinch test to assess which adjacent area has sufficient laxity and provides adequate soft tissue for closure in respect to relaxed skin tension lines.36 On the hand, a thin pliable skin flap is optimal to allow gliding and flexion of the underlying tendons.37 As geometric scar lines may not fall within relaxed skin tension lines, an aesthetically displeasing scar or decreased functional mobility may result if overlying a skin crease.38 This more advanced surgical closure may result in increased risk of postoperative complications, including bleeding, hematoma, ecchymosis or pain.16
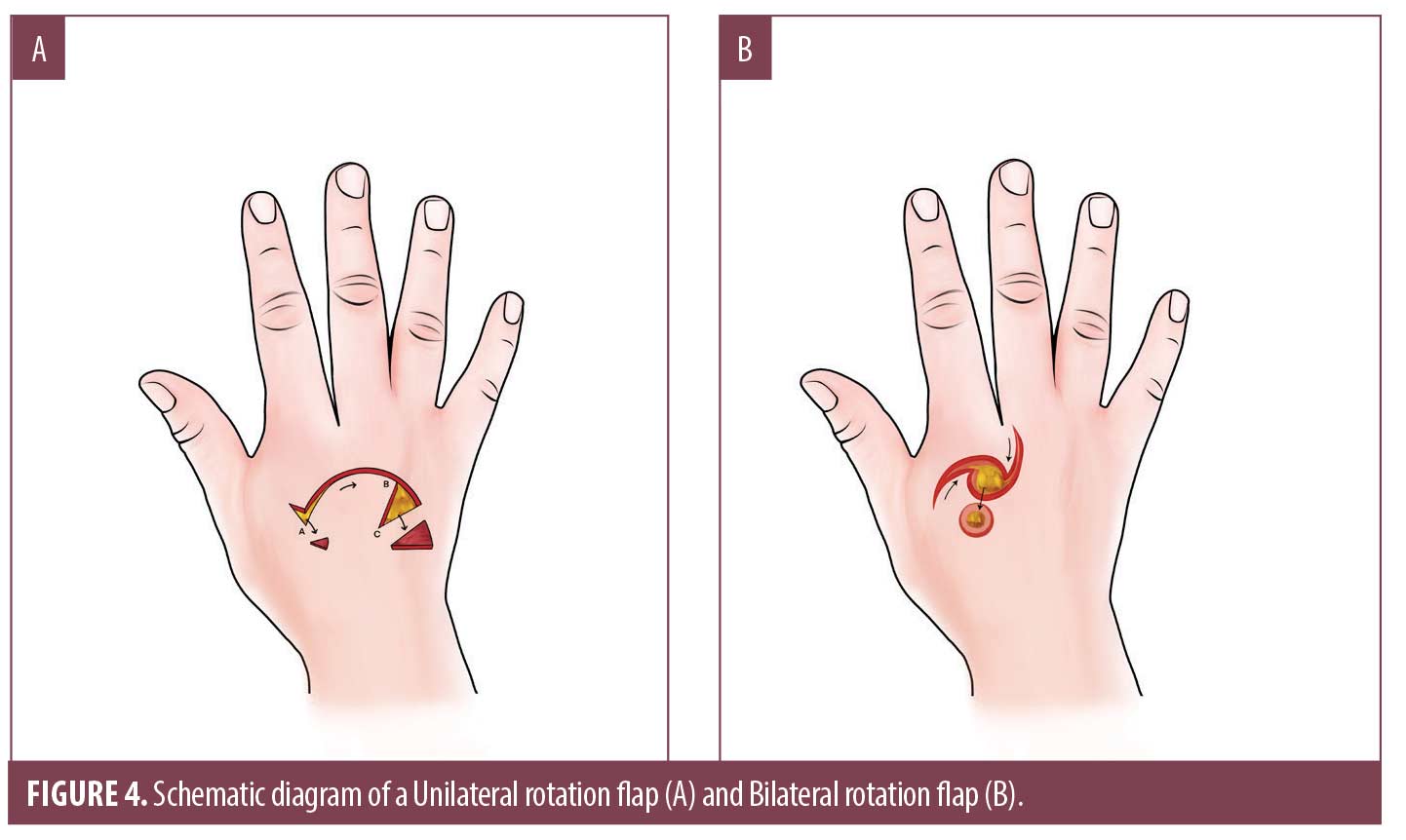
Rotation flaps slide adjacent tissue into the primary defect in a curvilinear motion, redistributing tension vectors along an arc adjacent to the primary defect over a larger surface area (Figure 4A, 4B).39 They provide suitable closures for dorsal hand defects up to 3 to 5cm in diameter and are often utilized when laxity is minimal prohibiting PLR.38 Single or bilateral rotation flaps may be used depending on the size of the defect and tissue laxity (Figure 4C, 4D). Maintaining adequate arterial blood supply and venous drainage, and preserving limb function are additional advantages to this closure.38 A common pitfall is a flap design that is too small, lacking sufficient rotational motion to close the defect.40 Because there is a loss of flap length and height when a unilateral flap is rotated onto the primary defect, the flap should be constructed three to four times larger than the diameter of the defect.36,38 If flap movement remains inadequate, a small back-cut or creation of a small Burow’s triangle can allow for increased motion.36
Advancement flaps employ a sliding motion and may be utilized for small to medium-sized defects on the dorsal hand that have exposed subcutaneous fat, tendon, or muscle and therefore are not ideal for secondary intention healing. Common advancement flaps on the hand include the Unilateral Burrow’s advancement (O to L) (Figure 5A, 5B), bilateral advancement (A to T) (Figure 5C, 5D) and the V-Y advancement (Figure 5E, 5F) [39]. These closures may utilize a local tissue reservoir contiguous to the defect or rely on a pedicle that can be advanced without disrupting blood supply. When possible, these flaps should be designed in a lateral-medial vector rather than superior-distal vector on the dorsal hand for optimal functional and cosmetic result. For larger defects, a vascular pedicle may not provide adequate blood supply to the distal edge of the flap, increasing risk of tissue necrosis.35
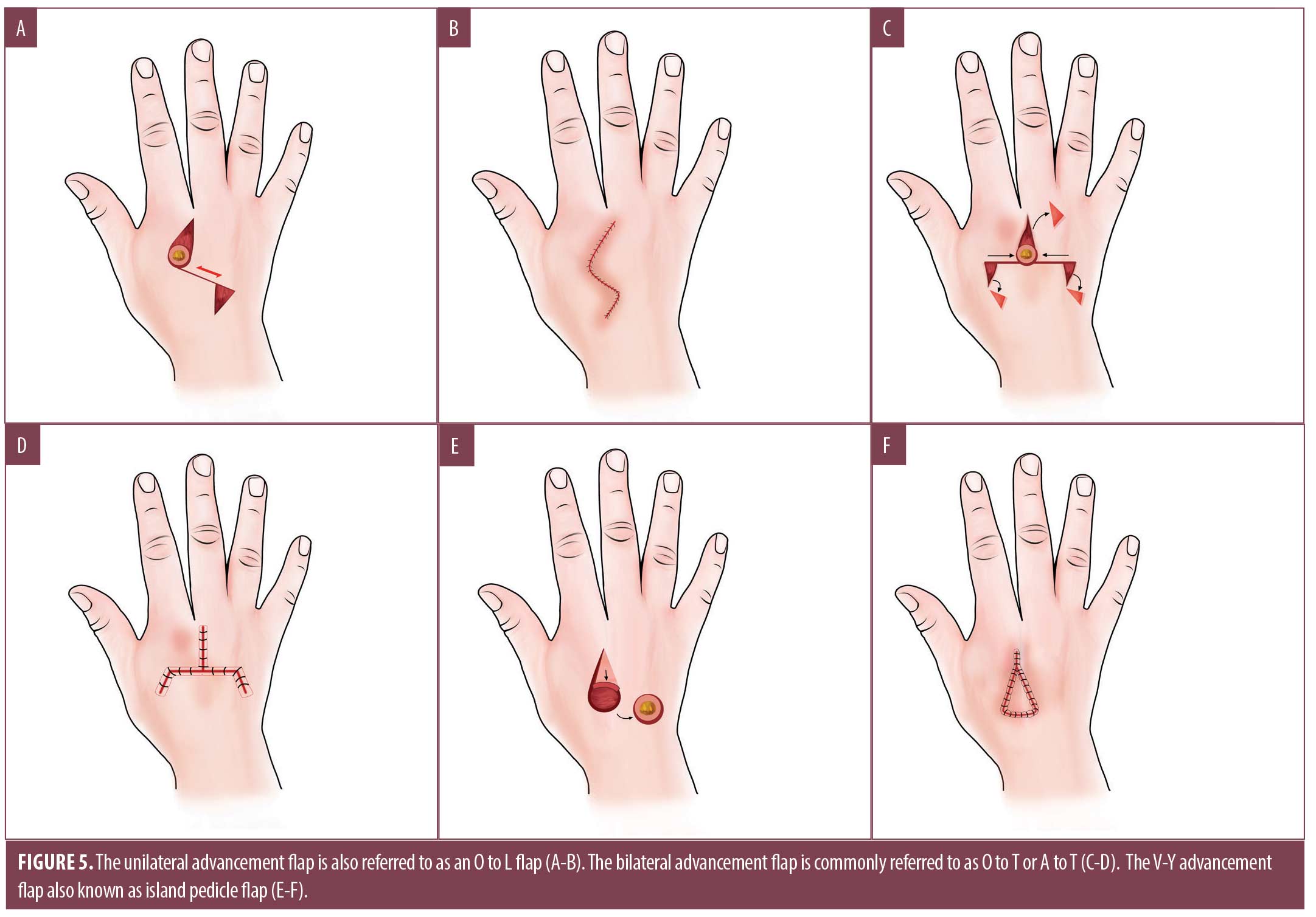
The V-Y advancement flap, also known as an island pedicle or kite flap, is particularly useful for distal fingertip defects not amenable to healing via SI or skin graft.36,39 It may also be considered for injuries involving the dorsal oblique or transverse distal fingertip with exposed bone.36,39 Its advantages include providing replacement of cutaneous tissue similar in color, texture, support, and sensation. On the distal phalanx, the V-Y flap incorporates the neurovascular bundle, preserving vascular and sensory supply to the tip of the finger.36 To perform the flap, the surgeon starts by drawing a V proximal to the primary defect. On the distal phalanx, the apex of the V should not extend beyond the DIP joint flexure, as this can result in contracture and functional impairment.36 Careful undermining in the subcutaneous plane, dissection from the flexor tendon sheath, and incisions down to the periosteum are performed to free the flap and advance it into position.36 Despite adequate undermining, only 1.0cm of flap mobilization is likely to be obtained on the distal phalanx, while V-Y advancement in areas with greater laxity (the dorsal hand) allow for more mobilization.36 After the tissue is advanced, the wound is closed in the form of a Y. Many surgeons advocate for hyper-eversion of the wound edges when suturing on fingers or hands to allow for proper wound healing.36
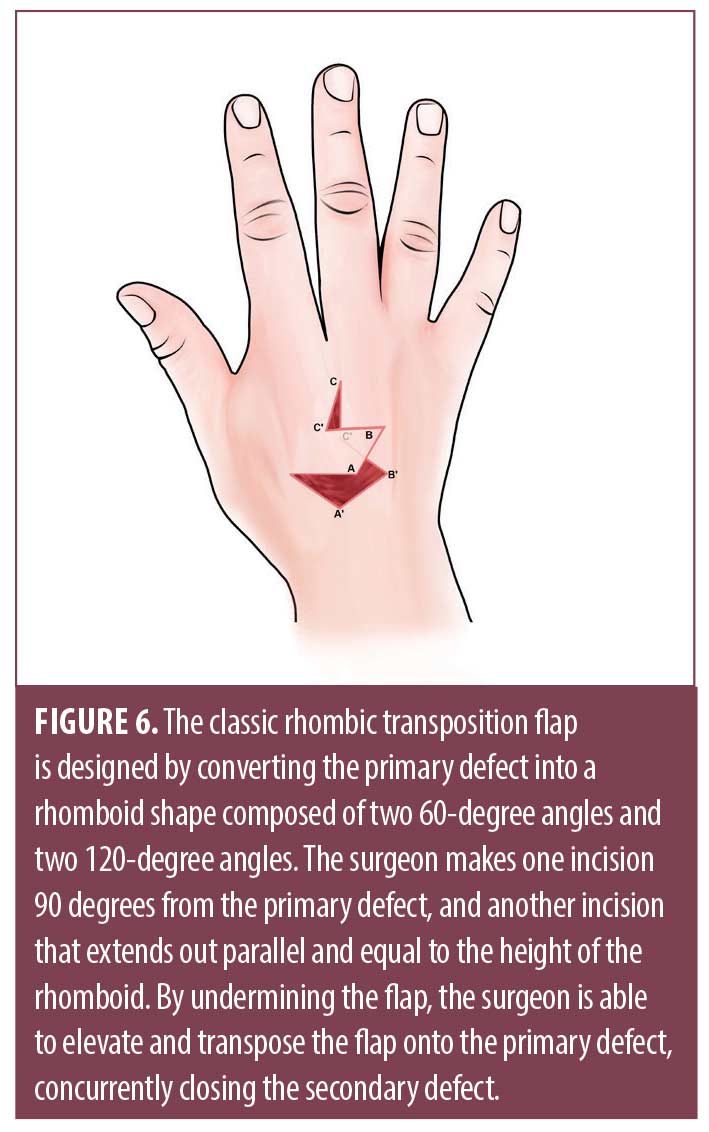
Transposition flaps use the primary motion of lifting local tissue reservoirs to repair defects with minimal laxity. The rhombic or Limberg flap uses a parallelogram with two 60-degree angles and two 120-degree angles (Figure 6).36 This flap is well suited to repair defects on the dorsal hand with minimal laxity such as overlying the MCP joints. The primary defect is converted to a rhomboid and the flap is designed by making an incision 90 degrees from the primary defect, ultimately removing a Burow’s triangle from the pivot point. The incision extends out parallel and equal to the height of the rhomboid. Undermining is performed to elevate and transpose the flap onto the primary defect, simultaneously closing the secondary defect.30 Modifications to the angles and shape of the flap may eliminate pointed edges, which have the potential for strangulation by tight skin closure.36 When designing the flap, surgeons should ensure the line of closure of the secondary defect is parallel to relaxed skin tension lines, thus reducing tension and redundant tissue puckering.36
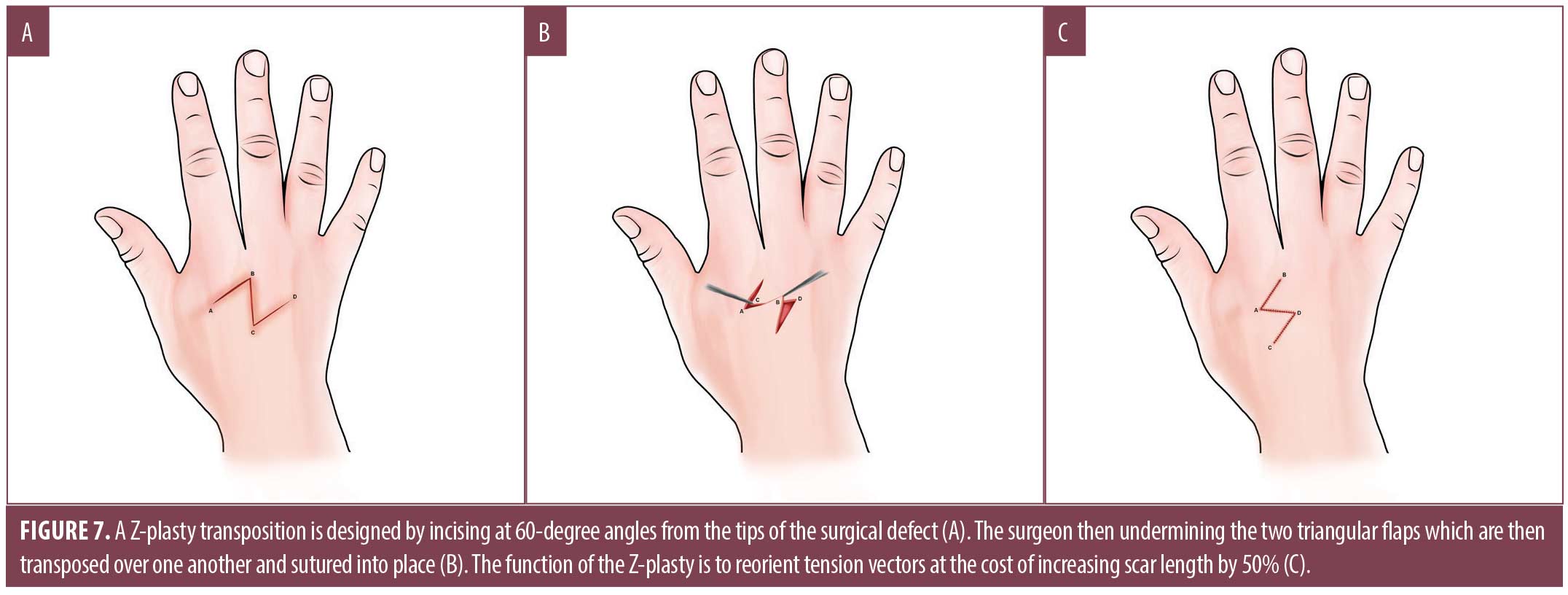
The Z-plasty is a transposition flap used for defect repair and to correct skin contractures. In general, incisions on the hand should be made parallel to major skin creases. Incisions made perpendicular will hypertrophy under tension, resulting in functional impairment as the scar contracts.7 When performing a Z-plasty, a 60-degree angle is commonly used from the tips of the surgical defect [wink]. After undermining, the two triangular flaps are transposed over one another and sutured into place. When used for scar revision, a Z-plasty can increase scar length by approximately 50 percent, whereby it allows reorientation of tension vectors; this may be helpful in hand and digit defects to reduce functional impairment when incisions cross volar creases (Figure 7).7
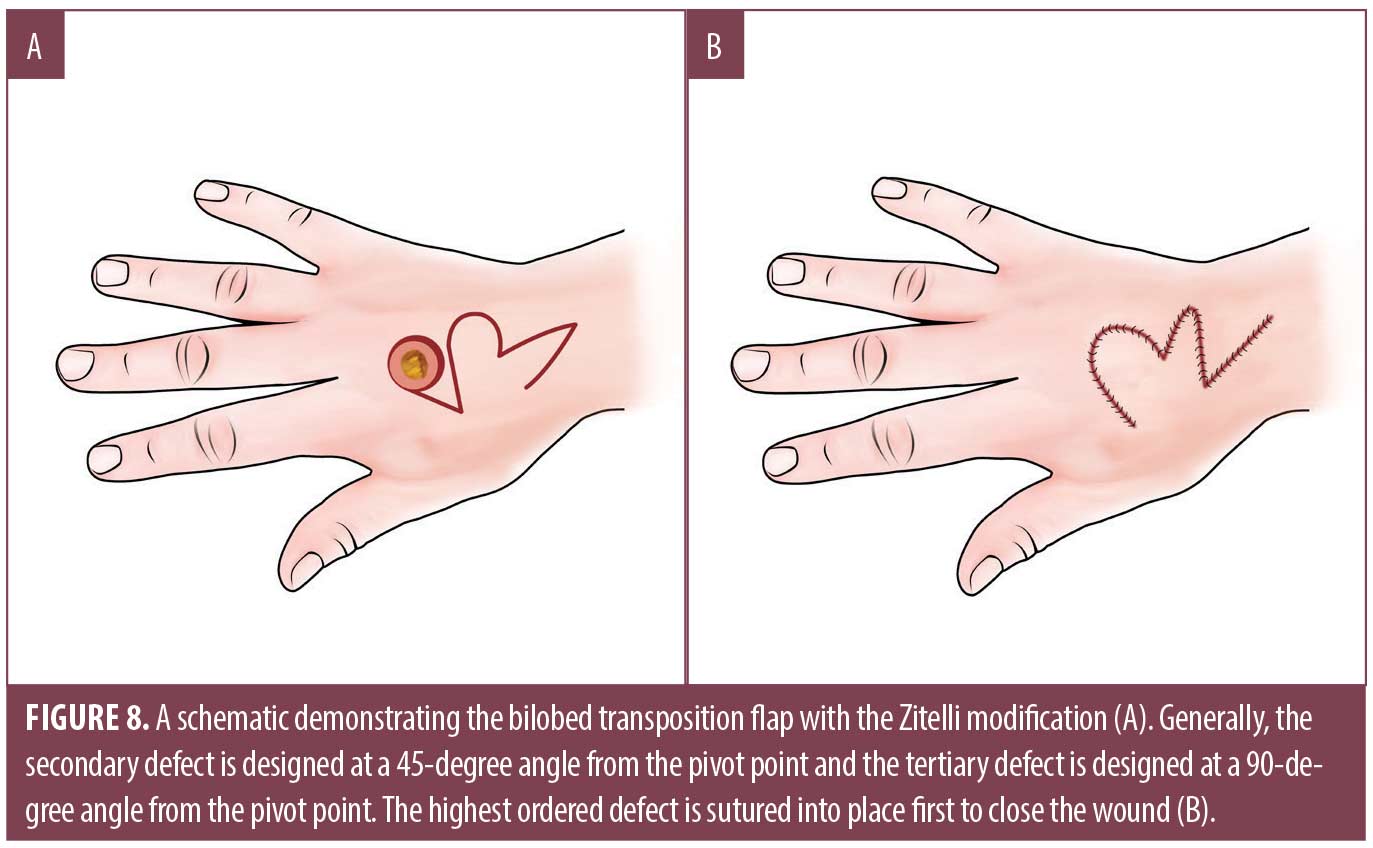
The modified bilobed design by Zitelli uses a multilobed transposition flap in areas where skin is less mobile, redistributing tension to areas of greater tissue laxity.41 It has an inherent Z-plasty-like design that maximizes rotation and minimizes tension compared with other rotational flaps.41 While the bilobed flap is often used on the nose or cheek, it has also been described on the dorsal hand and thumb where the thenar web space can be utilized when planning the first lobe.42,43 Classically when designing the flap, the primary lobe is equal in diameter to the size of the defect and the secondary lobe is equal to or slightly smaller than the primary lobe (Figure 8). However, on the dorsal hand, the secondary lobe may be considerably undersized compared to its nasal counterpart.41 The arcs of the bilobed flaps are constructed to allow for approximately 45 degrees of rotation for both the primary and secondary lobules.41 Overall, the flap should pivot at 90 to 110 degrees and standing cones should be excised at pivot points.41 The tertiary defect is closed first, reducing tension for optimal closure of the primary and secondary defects. The common complication of pincushioning seen in bilobed flaps has not been described on the hand. Ensuring appropriate flap size, performing sufficient undermining, and utilizing tacking sutures may safeguard against this issue. Its use may be limited by atrophic or actinically damaged skin, as the vascular supply of the pedicled flap may be insufficient.41
The fasciocutaneous sliding flap is useful for medium sized defects on the dorsal hand with exposed tendons, joints or neurovascular structures [wink]. Surgeons should design the flap in a fist position to provide an accurate measurement and anticipate post-operative tension.44 To design the flap, two lateral lines are drawn proximally from the widest portion of the defect towards the extensor wrist.39 The two lateral limbs are then connected by a third line, creating a rectangular flap. If the defect is under tension, an arciform or keystone variation may allow better recruitment of surrounding tissue with a V-Y closure at the proximal ends.39–44 After designing the flap, one lateral digit is incised down to the dorsal superficial fascia. Incisions can be extended to the dorsal deep lamina, superior to the paratenon and dorsal deep fascia, to gain additional mobility and access the fascial plane for undermining.39–44 Once the flap is released from the paratenon on the desired digit, superficial undermining in the subcutaneous fat should be performed on the remaining digits to mobilize the fascial pedicle; the flap will then slide into place and be sutured in a tension free manner.44
Vascular supply derived from dorsal perforating arteries is one key advantage to the fasciocutaneous sliding flap. The parallel arrangement of the dorsal arterial network and its perforators provide ample blood supply, reducing the risk of ischemia compared to random pattern flaps based on the dermal plexus.44 Communication between the dorsal and palmar systems typically provides further blood flow to the fasciocutaneous sliding flap.44 Redistribution of tension and a broad flap design afford other advantages, as distant tissue reservoirs redistribute tension, minimizing limitations on joint mobility.44 This single-staged reconstruction with a local tissue donor site is optimal in terms of color, texture and sensation, while minimizing donor site morbidity, sensory deficits and aftercare restrictions.
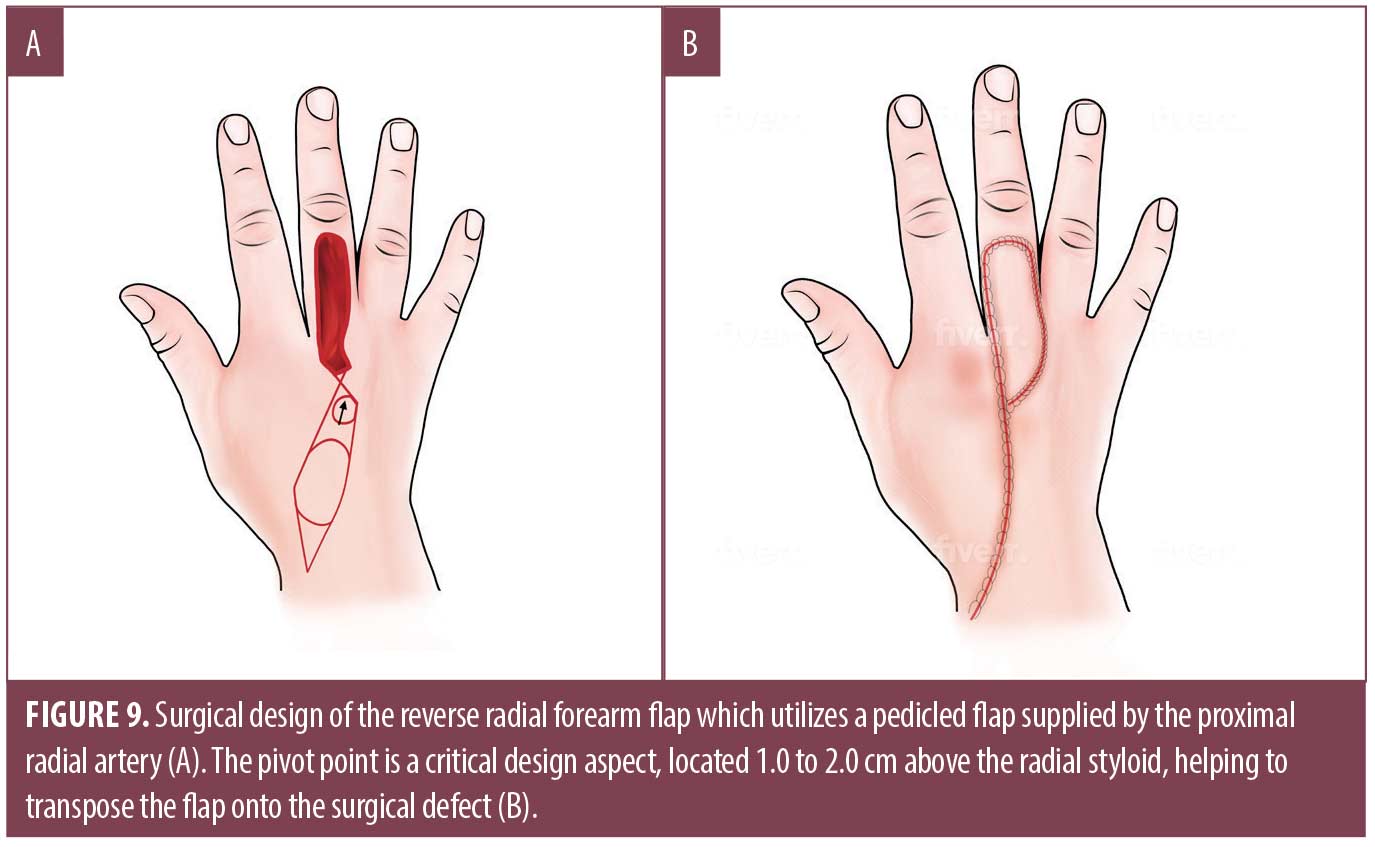
Regional flaps. The reverse radial forearm flap (RRFF), a variant of the radial forearm flap (RFF), is a complex pedicle axial pattern flap used for small to medium-sized defects on both the volar and dorsal surfaces of the hand (Figure 9A).37,38,45,46 General anesthesia is often required given the complexity of repair. Before harvesting the flap, doppler ultrasound should be performed to map the course of the radial artery. Careful measurement and marking of the defect and donor sites are made, often over the middle third of the forearm. A tourniquet is applied prior to incising the margins of the flap, extending distally to the proximal wrist crease, incorporating a pedicle overlying the course of the radial artery.47 The incisions should be made down to fascia, identifying the lateral intramuscular septum which contains the radial artery and veins.47 The inferior portion of the septum should be transected to allow elevation of the vascular pedicle.47 Careful dissection of the deep septum is continued proximally, ensuring proper depth near the radial margin of the flexor carpi radialis tendon. Transection of the vertical septum is most susceptible at this location and may result in separation of the flap from the blood supply.47 Critical structures such as the radial neurovascular bundle and the lateral antebrachial cutaneous nerve should be identified and preserved.47 After careful dissection beneath the deep fascia, the flap is raised ulnar to radial and rotated over the dorsal aspect of the wrist to the recipient site, relying on the distal radial artery for blood supply.37 To reduce the potential of vascular compression, a subcutaneous tunnel can be performed from the radial styloid to the defet or the incision can be extended to connect the radial styloid edge of the flap to the most proximal radial edge of the defect.47 Finally, the tourniquet is removed and adequate blood flow visualized or confirmed via doppler ultrasound before flap inset and suture placement.
The RRFF is more advantageous than the RFF because it is harvested without a skin paddle, allowing direct closure of the donor site.47 This flap requires ligation of the proximal radial artery, thus relying on patent ulnar to radial artery communications.48 The RRFF is contraindicated in patients with compromised vasculature; therefore, the Allen test should be performed preoperatively to ensure adequate collateral blood flow.47 The pivot point is key to designing this flap, which is located 1.0 to 2.0cm above the radial styloid (Figure 9B).47 Disadvantages to using this flap include poor aesthetic outcomes due to poor color matching. It also results in a large conspicuous donor site, often requiring a STSG. In addition, it requires sacrifice of a major vessel to the hand.37 The RRFF is also not ideal if delayed reconstructive procedures are planned. Meticulous post-operative care to the flap and skin graft are critical to ensure successful healing. Failure is commonly attributed to technical error in elevation or in setting of the flap.47 Venous congestion, more than arterial insufficiency, is another complication that may result in partial or total flap loss.
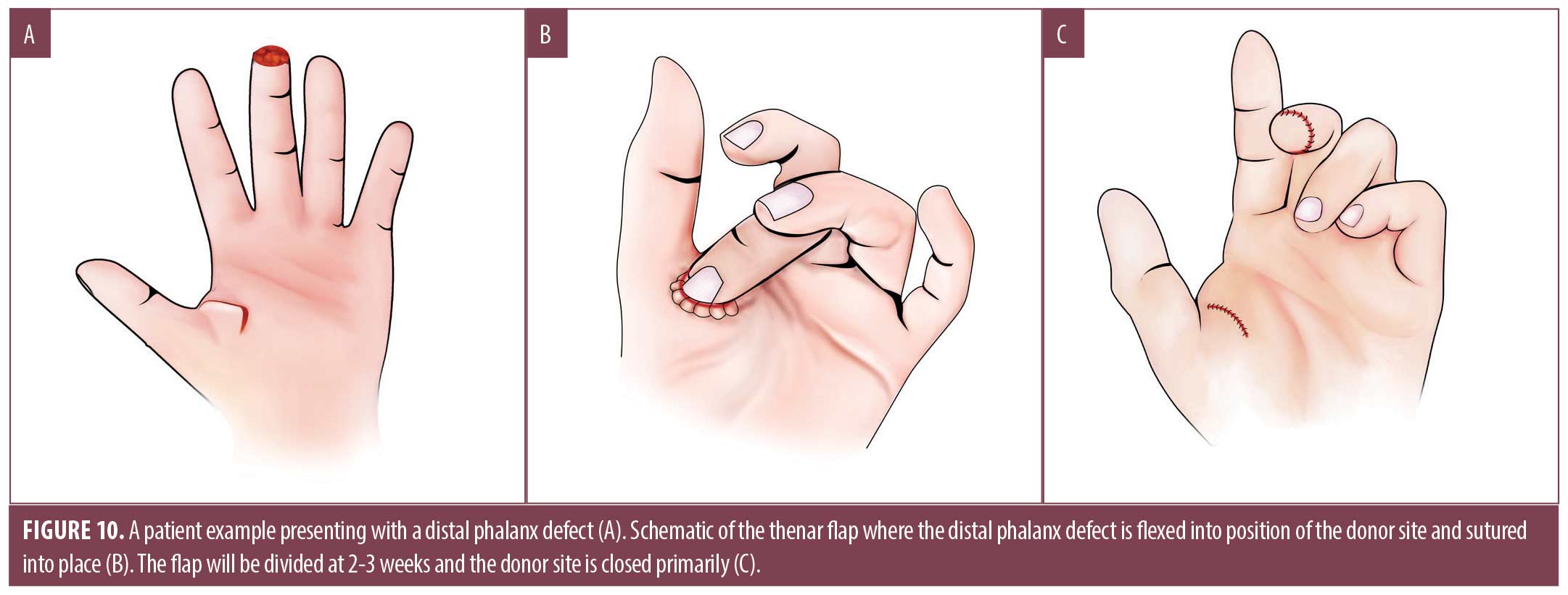
Interpolation flaps. An interpolation flap is a two-staged technique in which the flap base is noncontiguous to the defect and are used when there is either inadequate local tissue or mobility. Interpolation flaps can either be axial flaps named for specific arterial supply, or random pattern flaps that rely on a vascular plexus. The thenar flap is a two-step closure suitable for volar phalanx defects that utilizes a full thickness flap from the thenar eminence (Figure 10A).38 Advantages are similar to those of the V-Y advancement for the distal phalanx. Its main disadvantage is frequent formation of PIP joint contracture or stiffness.36 To execute, the finger is flexed to contact the thenar eminence. The point of contact at the donor site is level with the MCP; more proximal locations may result in excessive tension and flap failure.36 The donor site is designed as a rhombic, circular or H-shaped flap, and its size should be slightly larger than the defect.36,37 Care is taken to avoid the nearby neurovascular bundle, specifically the radial digital nerve of the thumb, as the flap is elevated from the thenar muscle fascia.36,38 This is achieved through blunt undermining in the fascial plane, being mindful of the minimal subcutaneous tissue layer on the dorsum of the hand.48 The finger is flexed into position so that the primary defect is in contact with the newly created thenar flap (Figure 10B). The finger is left in place for 2 to 3 weeks before being divided. After the flap is divided (Figure 10C), the donor site can be closed primarily or let to heal by SI. Many surgeons then place petroleum impregnated gauze under or around the base of the flap to provide structure and antibacterial protection.45,50
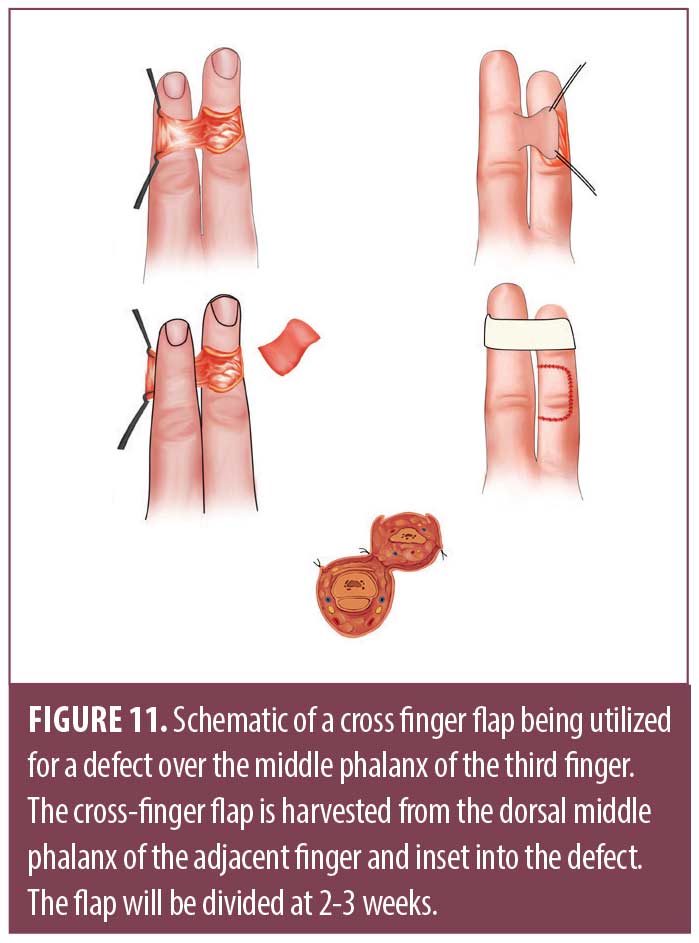
The cross-finger flap is another two-step reconstruction for volar soft tissue defects on the middle or distal phalanx.30,32,33 Classically, the donor finger will be radial to the finger with the surgical defect, although the middle finger may be used to repair a defect on either side.36,38,39 The flap is drawn as a rhomboid or rectangle on the donor finger that slightly larger than the surgical defect (Figure 11). This shape is dissected to the level of subcutaneous tissue, essentially creating a flap rotated 180 degrees around its hinge point. This flap is then transposed in an open book fashion onto the finger with the surgical defect, covering the paratenon of the extensor tendon.36,38,39 Lastly, a FTSG is taken elsewhere to close the secondary defect. Similar to the thenar flap, the fingers are immobilized for 2 to 3 weeks. The main disadvantage is PIP joint stiffness due to immobilization.36,39
Conclusion
The goals of cutaneous surgery on the hand include conserving native anatomy and avoiding surgical complications, such as hematoma, infection, functionally debilitating contractures, motor, or sensory nerve damage. The review detailed above discusses repair options for this anatomic location, given the unique and challenging objective of retaining hand and digit function. Lastly, the high prevalence of keratinocyte carcinoma on the dorsal hand highlights the need for clinical studies that compare surgical repairs and their outcomes on this anatomic area following dermatologic surgery.
References
- Maciburko SJ, Townley WA, Hollowood K, et al. Skin cancers of the hand: a series of 541 malignancies. Plast Reconstr Surg. 2012;129(6):1329–1336.
- Loh TY, Rubin AG, Brian Jiang SI. Basal Cell Carcinoma of the Dorsal Hand: An Update and Comprehensive Review of the Literature. Dermatol Surg. 2016;42(4):464–470.
- Mull JL, Phelan PS, Mull AB, et al. Squamous Cell Carcinoma of the Hand: A Retrospective Study in Immunosuppressed and Immunocompetent Individuals. Dermatol Surg. 2020;46(8):1014–1020.
- Ruffolo AM, Sampath AJ, Kozlow JH, et al. Melanoma of the Hands and Feet (With Reconstruction). Clin Plast Surg. 2021;48(4):687–698.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: A prospective multicenter study. J Am Acad Dermatol. 2019;81(3):767–774.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-Sparing Mohs Surgery for Melanoma. Dermatol Surg. 2016;42(1):83–93.
- Rohrer TE, et al. Dermatologic Surgery of the Hand: General Principles and Avoiding Complications. The Journal of Dermatologic Surgery and Oncology. 1994 Jan;20(1):19–34;
- Mack GR. Superficial anatomy and cutaneous surgery of the hand. Adv Dermatol. 1992;7:315–352.
- de-Ary-Pires B, Valdez CF, Shecaira AP, et al. Cleland’s and Grayson’s ligaments of the hand: a morphometrical investigation. Clin Anat. 2007;20(1):68–76.
- Chandrasoma J, Harrison TK, Ching H, et al. Peripheral Nerve Blocks for Hand Procedures. N Engl J Med. 2018;379(10):e15.
- Kachare SD, Meredith LT, Kachare MD, et al. Surface Landmarks to Provide a Safe Ulnar Nerve Block in the Wrist: Anatomical Study and Literature Review. Eplasty. 2020;20:e12. Published 2020 Oct 19.
- Eming SA. “Biology of Wound Healing.” Dermatology, edited by Jean Bolognia et al., 4th ed., Elsevier, 2018, pp. 2413–2422.
- Wright DR, Kwak Y, Goldberg LH, et al. Repair of a Large Dorsal Hand Defect After Mohs Micrographic Surgery. Dermatol Surg. 2020;46(3):413–415.
- Etzkorn JR, Sobanko JF, Shin TM, et al. Repair of a Large Dorsal Hand Defect With Exposed Tendons. Dermatol Surg. 2018;44(12):1583–1586.
- Snow SN, Stiff MA, Bullen R, et al. Second-intention healing of exposed facial-scalp bone after Mohs surgery for skin cancer: review of ninety-one cases. J Am Acad Dermatol. 1994;31(3 Pt 1):450–454.
- Bosley R, Leithauser L, Turner M, et al. The efficacy of second-intention healing in the management of defects on the dorsal surface of the hands and fingers after Mohs micrographic surgery. Dermatol Surg. 2012;38(4):647–653.
- Magliano J, Rossi V, Turra N, et al. Secondary-intention healing following Mohs micrographic surgery for squamous cell carcinoma of a finger. Int Wound J. 2019;16(3):860–861.
- Lateo SA, Langtry JA. A prospective case series of secondary intention healing for surgical wounds on the dorsum of the hand. Clin Exp Dermatol. 2013;38(6):606–611.
- Zitelli JA. Secondary intention healing: an alternative to surgical repair. Clin Dermatol. 1984;2(3):92–106.
- Schimmel J, Belcher M, Vieira C, et al. Incidence of Surgical Site Infections in Second Intention Healing After Dermatologic Surgery. Dermatol Surg. 2020;46(12):1492–1497.
- Miedema J, Hsia LB, Varma R. Wound Healing on the Dorsal Hands: An Intrapatient Comparison of Primary Closure, Purse-String Closure, and Secondary Intention. Cutis. 2021;107(6):318–319.
- Hansen T, Gangal A, Hijab E, et al. Postoperative surgical site infection rate in patients with diabetes following Mohs micrographic surgery: a retrospective analysis [published online ahead of print, 2022 Feb 5]. J Eur Acad Dermatol Venereol.
- Goldberg LH, Alam M. Elliptical excisions: variations and the eccentric parallelogram. Arch Dermatol. 2004;140(2):176–180.
- Brandão CM, Weimann ETS, Terzian LR, et al. Keep it simple. A ten-year experience in reconstructions after Mohs micrographic surgery. An Bras Dermatol. 2020;95(6):714–720.
- Kang AS, Kang KS. A Systematic Review of Cutaneous Dog Ear Deformity: A Management Algorithm. Plast Reconstr Surg Glob Open. 2020;8(9):e3102. Published 2020 Sep 23.
- Joo J, Custis T, Armstrong AW, et al. Purse-string suture vs second intention healing: results of a randomized, blind clinical trial. JAMA Dermatol. 2015;151(3):265–270.
- Cohen PR, Martinelli PT, Schulze KE, et al. The cuticular purse string suture: a modified purse string suture for the partial closure of round postoperative wounds. Int J Dermatol. 2007;46(7):746–753.
- Hassanpour SE, Hosseini SN, Abdolzadeh M. Purse-string suture as a complementary technique with conventional flaps in repairing fingertip amputation. Tech Hand Up Extrem Surg. 2011;15(2):94–98.
- Manna MLQ, Ucelli JLR, Ajuz NCC, et al. Reconstruction of surgical defect of the dorsum of the hand. An Bras Dermatol. 2017;92(5 Suppl 1):132–134.
- Rehim SA, Chung KC. Local flaps of the hand. Hand Clin. 2014;30(2):137-v.
- Das De S, Sebastin SJ. Considerations in Flap Selection for Soft Tissue Defects of the Hand. Clin Plast Surg. 2019;46(3):393–406.
- Biswas D, Wysocki RW, Fernandez JJ, et al. Local and regional flaps for hand coverage. J Hand Surg Am. 2014;39(5):992–1004.
- Wink J, Gandhi R, Ashley B, et al. Flap Reconstruction of the Hand. Plastic and Reconstructive Surgery. 2020; 145(1): 172e–183e.
- Maksoud, Ahmed M. Al, and Iftikhar Ahmed. Rotation Flap to Cover a Large Defect on the Dorsum of the Hand. Journal of Surgical Case Reports. 2015(11): rjv139.
- Ricks M and Cook J. Extranasal Applications of the Bilobed Flap. Dermatologic Surgery. 2005 Aug;31(8 Pt 1):941–948.
- Agdoğan, Ö. and Karaçal, R. Bilobed Flap for Reconstruction of a Large Keratoacanthoma of the Thumb. Case Reports in Plastic Surgery and Hand Surgery. 2019; 6(1):86–87.
- Moy RL, Quan MB. The presence of human papillomavirus type 16 in squamous cell carcinoma of the proximal finger and reconstruction with a bilobed transposition flap. J Dermatol Surg Oncol. 1991;17(2):171–175.
- Sobanko JF, Fischer J, Etzkorn JR, et al. Local fasciocutaneous sliding flaps for soft-tissue defects of the dorsum of the hand. JAMA Dermatol. 2014;150(11):1187–1191.
- Rohrer, Thomas E, et al. Staged Interpolation Flaps. Flaps and Grafts in Dermatologic Surgery, Elsevier. 2018, pp.116–131.
- Aizman L, Perz AM, Lukowiak TM, et al. Reverse Dorsal Metacarpal Artery Flaps to Repair Distal Hand and Dorsal Finger Defects After Mohs Micrographic Surgery. Dermatol Surg. 2021;47(8):1130–1132.
- Kaufman MR, Jones NF. The reverse radial forearm flap for soft tissue reconstruction of the wrist and hand. Tech Hand Up Extrem Surg. 2005;9(1):47–51.
- Etzkorn JR, Goldbach HS, Sobanko JF, et al. Repair of a Deep Defect on the Proximal Phalanx. Dermatol Surg. 2019;45(10):1315–1318.
- Miller CJ, Sobanko JF, Albertini JG. Axial Pattern and Interpolation Flaps. In: Avram MR, Avram MM, Ratner D. eds. Procedural Dermatology. McGraw Hill; 2015. Accessed October 17, 2021.
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: Part I. Cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72(3):377–387.
- Rohrer, Thomas E, et al. Skin Grafts. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier, 2018, pp. 132–144.
- Gloster HM Jr, Daoud MS, Roenigk RK. The use of full-thickness skin grafts for the repair of defects on the dorsal hand and digits. Dermatol Surg.1995;21(11):953–959.
- Koob TJ, Rennert R, Zabek N, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10(5):493–500.
- Sheikh ES, Sheikh ES, Fetterolf DE. Use of dehydrated human amniotic membrane allografts to promote healing in patients with refractory non healing wounds. Int Wound J. 2014;11(6):711–717.
- Wisco OJ. Case series: The use of a dehydrated human amnion/chorion membrane allograft to enhance healing in the repair of lower eyelid defects after Mohs micrographic surgery. JAAD Case Rep. 2016;2(4):294–297. Published 2016 Jul 27.
- Yang YW, Ochoa SA. Use of Porcine Xenografts in Dermatology Surgery: The Mayo Clinic Experience. Dermatol Surg. 2016;42(8):985–991.

