 J Clin Aesthet Dermatol. 2022;15(9):16–19.
J Clin Aesthet Dermatol. 2022;15(9):16–19.
by Mark Nestor, MD, PhD; David Pariser, MD; James Del Rosso, DO; Dee Anna Glaser, MD; David Goldberg, MD, JD; Glynis Ablon, MD; Z. Paul Lorenc, MD; and Julie Santos, PA-c
Drs. Nestor and Santos are with the Center for Clinical and Cosmetic Research in Aventura, Florida. Dr. Nestor is additionally with the Department of Dermatology and Cutaneous Surgery, Department of Surgery, at the University of Miami, Miller School of Medicine in Miami, Florida. Dr. Pariser is with Virginia Clinical Research, Inc., in Norfolk, Virginia. Dr. Del Rosso is with JDR Dermatology Research in Las Vegas, Nevada, and Advanced Dermatology and Cosmetic Surgery in Maitland, Florida. Dr. Glaser is with the Department of Dermatology, Otolaryngology, and Internal Medicine at Saint Louis University School of Medicine in St. Louis, Missouri. Dr. Goldberg is with Schweiger Dermatology Group in New York, New York. Dr. Ablon is with the Ablon Skin Institute & Research Center in Manhattan Beach, California. Dr. Lorenc is with the Lorenc Aesthetic Plastic Surgery Center in New York, New York.
FUNDING: Financial support was provided by The Vial Dermatology CRO in San Francisco, California.
DISCLOSURES: The authors report no conflicts of interest relevant to the content of this article.
ABSTRACT: Objective. Dermatology and aesthetic medicine has experienced significant growth in recent years, driven by industry-sponsored research performed by clinical investigators. Contract research organizations (CROs) play an important role to ensure clinical trials are run efficiently, ethically, and according to Good Clinical Practice. An advisory group of dermatologists and aesthetic researchers was assembled to obtain feedback about CRO experiences for developing a “next-generation” specialty CRO for dermatology and aesthetic medicine.
Methods. Experienced dermatologists and aesthetic physician researchers convened during the 2022 Winter Dermatology Annual Meeting in Kauai, Hawaii, to discuss experiences and make suggestions regarding CROs. Topics included positive and negative aspects of CRO experiences, desirable CRO services, and how CROs can be improved.
Results. Benefits of working with CROs include project, data, and resource management and availability of technology. Desired functions include rapid study start-up, subject recruitment, and accurate and organized site-related documentation. Other qualities include access to large subject populations close to study locations, use of CRO-based clinical research assistants to support principal investigators across study sites, and scientific consultation, protocol development, medical writing, project management, clinical and medical monitoring, data management, biostatistics, and pharmacovigilance.
Conclusion. CROs serve a vital role in the development process of drug, device, and therapeutics; however, it is important that changes to traditional CRO models are made to provide improved interactions with researchers in dermatology and aesthetic medicine.
Keywords: CRO, contract research organization, research, trial, clinical, dermatology, investigator
The field of dermatology has experienced considerable growth in recent years. Reasons for this include the increase in the geriatric population, the rising prevalence of dermatologic disorders, and increasing awareness of skin problems via the internet and social media.1 For example, the overall incidence of basal cell carcinoma increased by 145 percent from 2000 to 2010, and the overall incidence of squamous cell carcinoma increased 263 percent over that same period.2 In South Florida, the incidence of treated nonmelanoma skin cancer was 466.5 per 100,000 annually among people aged 65 years old or younger in 2021, but 10,689.8 per 100,000 among people older than 65 years.3 Efforts to meet these growing needs have resulted in a vastly expanded arsenal of medications and technologies, increasing market growth from approximately $21.5 billion in 2017 to an estimated $34.5 billion by 2023.1
Similarly, there has been explosive growth in the field of aesthetic medicine following the development of numerous technologies, such as minimally invasive, high intensity focused ultrasound for skin rejuvenation and laser devices for body contouring. This growth is also driven by social media4 and an increase in disposable income. Since 2000, the use of botulinum toxin and laser skin resurfacing procedures have increased by 845 percent and 248 percent, respectively, and cosmetic procedures overall have increased by 163 percent.5 According to a survey by the American Society for Dermatologic Surgery, the patient population considering a cosmetic procedure has more than doubled from 30 percent in 2013 to 70 percent in 2017. The most common procedures are light and laser therapy, facial rejuvenation injections, chemical peels, and body sculpting.6
Most of these advances in dermatology and aesthetic medicine have been driven by industry-sponsored research performed by clinical investigators. Contract research organizations (CROs) have become an integral part of research across many clinical disciplines, including the pharmaceutical, biotechnology, and medical device industries. It is the responsibility of CROs to act on behalf of study sponsors to ensure clinical trials are run efficiently, ethically, and according to Good Clinical Practice.7 They have evolved from providing research animals in the 1940s and 1950s to specializing in preclinical and clinical testing and can assist in carrying out research trials without the need for sponsors to hire additional permanent staff.8
Within the pharmaceutical industry, CROs were originally formed to assist in bringing new medicines to market. The growth of CROs rapidly grew following increased regulations by the Food and Drug Administration (FDA). Changes to the Federal Food, Drug and Cosmetic Act, known as the Kefauver Harris Amendment or “Drug Efficacy Amendment,” was signed into law by President Kennedy on October 10, 1962.9 The Amendment introduced legislation specifically designed to prevent a repeat of the thalidomide tragedy. Among other things, the amendments required that drug manufacturers must prove the effectiveness of their products prior to marketing and to report serious post-marketing adverse events.10 Evidence of drug efficacy must now be based on adequate and well-controlled clinical trials that are conducted by qualified experts and require study subjects to provide informed consent. It also introduced the phase sequence of drug development.9
Currently, CROs have become a large competitive, profitable industry responsible for monitoring clinical trials and ensuring site compliance with protocols via in-person and remote monitors; managing regulatory documents and clinical data collected during studies; liaising between sponsors, clinical sites, and vendors; and mitigating problems that may arise during the study. There are currently 4,250 CROs in the United States as of 2022 making it difficult for sponsors to choose a CRO and for research sites to give adequate input for optimal partnerships.11 The ideal CRO will have experience in the relevant areas of study, a proven track record launching new drugs and devices, the skills and knowledge to take a drug or device through each phase of the drug development process, and experience in communicating with regulatory agencies. Some study sponsors and many clinical research sites, however, believe CROs may have become a potential encumbrance instead of a catalyst for research and that it is time for CROs to change.
In order to explore innovations and needed changes in the evolving CRO marketplace, a high level advisory group of top dermatologists and aesthetic researchers was assembled to obtain feedback about their prior and current CRO experiences to further develop a “next-generation” specialty CRO for dermatology and aesthetic medicine. The group was asked to give feedback and advice to a newly formed CRO (Vial Health Technology, Inc.; San Francisco, California).
Methods
The panel of expert dermatologists and aesthetic physicians convened in person and virtually during the 2022 Winter Dermatology Annual Meeting in Kauai, Hawaii, to discuss their experiences and make suggestions regarding current CROs. All of the clinical participants were experienced researchers who have previously worked with multiple CROs. Key topics for discussion included: positive and negative aspects of CRO experiences, services should CROs focus on, how CROs can be improved, and what can be done to implement changes. These topics were sent to participants for their consideration in advance of the meeting. A moderator used a PowerPoint presentation to lead the discussion, which was recorded and later transcribed.
Discussion
The discussion began with introductory comments from the moderator, which included a review of the agenda and introductions between the six CRO members and eight clinician researcher participants. The discussion began with positive aspects of prior CRO experiences.
Benefits of the CROs of today. The study participants acknowledged the potential benefits of working with a CRO, such as project, data and resource management, and the availability of the most sophisticated technology. Specific positive CRO experiences included warnings about impending expirations of institutional Review Board (IRB) approvals, Good Clinical Practice (GCP) and Clinical Laboratory Improvement Amendments (CLIA), and medical licenses. They can also share knowledge about common problems between clinical trial sites and the means for their resolution.
Problems with CROs of today. There were numerous concerns with existing CROs. Many of these issues are regarding clinical research associates (CRAs) or study monitors, that are too few in number, sometimes poorly trained and do not respond to researchers as needed in a timely manner, and the lack of alignment between the CRA, CRO, and sponsor. Other concerns are high turnover rates of CRO personnel leading to poor communication and follow up. Other concerns were electronic data capture (EDC) systems not matching source documents, the lack of assistance during FDA audits, payment delays, and difficulty contacting CRO finance teams.
On what areas should the CROs of today focus? It was determined that there are several areas where CROs should focus their expertise. These include rapid initiation and execution of clinical trials, use of the best available technology, clear and continuous communication, and resource management. Consequently, there are several areas where the CROs of today can be improved. These include more rapid start-up time, improved patient recruitment, better data analysis, greater CRA or monitor capabilities, and more rapid communication. It is essential that CROs are equipped with the most current EDC and clinical trial management systems (CTMS). For researchers, changes may be encouraged by requesting referral to preferred CROs from the sponsor and providing pointed feedback to CROs.
What do sponsors need? The CRO acts on behalf of the sponsor to make sure the study is run efficiently, ethically, and according to Good Clinical Practice.12 Additionally, it is essential that the CRO acts as a liaison between the sponsor, the clinical trial site, and any vendors by providing consistent, seamless communication. Specifically, the ideal CRO from the sponsors perspective would quickly initiate a trial with model research sites that can quickly enroll per the protocol, efficiently manage any updates and changes to the protocol, provide an ideal electronic data capture system, monitor sites effectively, and provide assistance when necessary. Other needs of the sponsor include a CRO that can maintain agreed-upon protocol budgets, precise data analysis that are consistent with source documents, and managing prompt payments to clinical trial sites and vendors. Sponsors also want to pay as little as possible for these services.
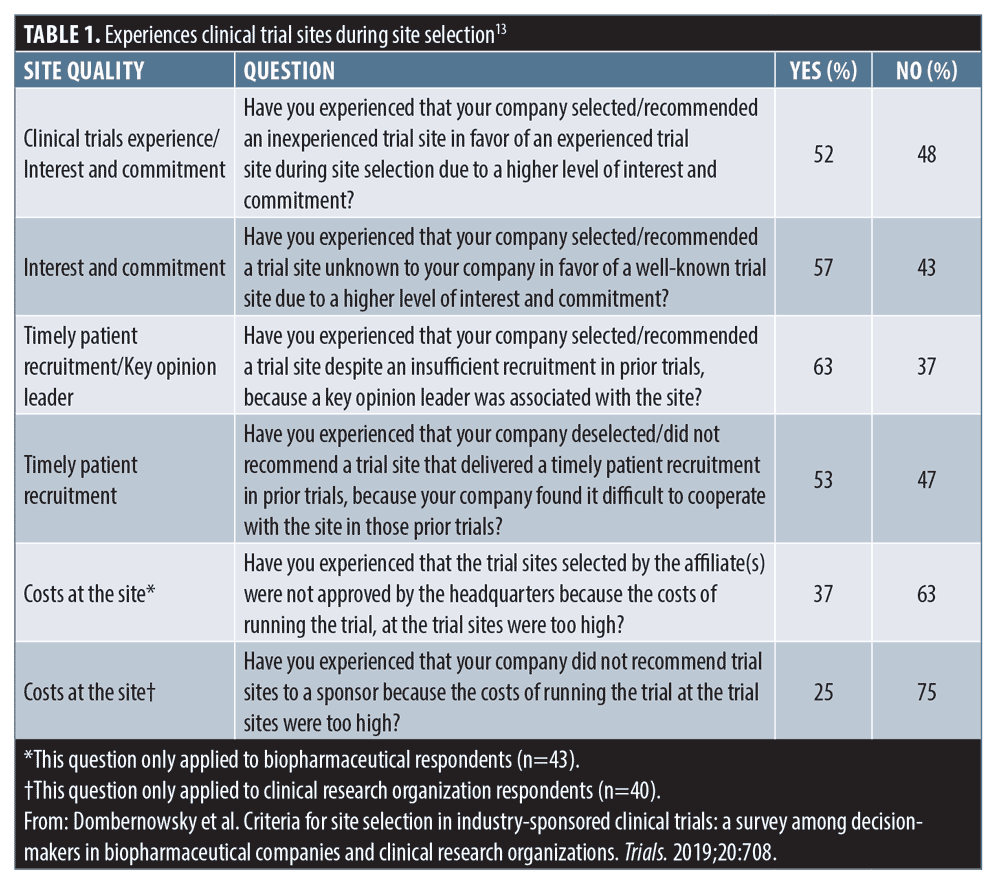
Desired clinical trial site attributes. Study sponsors and CROs also have expectations for clinical study sites. A survey of Nordic multinational biopharmaceutical companies (n=20) and CROs (n=23) identified the relative importance of site-related qualities.13 Among the total responses received (N=101), most (88%) preferred reaching enrollment goals at trial 10 percent faster versus cutting costs at all sites by 20 percent. Most respondents (75%) agreed that they would be interested in cooperating with an inexperienced trial site if the site had access to a large patient population and would choose an inexperienced trial site in favor of an experienced site due to a higher level of interest and commitment (52%) (Table 1). Survey respondents preferred trial sites that were best at having the first subjects ready for inclusion immediately after site initiation versus having good data entry (42%), had good documentation and reporting practice (25%), had easily reachable site personnel and backup (23%), fast contractual procedure times (6%), a key opinion leader associated with the site (3%), and updated equipment and facilities (1%).13 By using a superior CRO, trial sites should not have to sacrifice expense for rapid enrollment or good data entry for rapid site initiation.
The future of CROs. A primary goal of the next-generation CRO is to start with the latest available technology, listen to the concerns of clinical research sites, and provide them with a partner who can provide a seamless conduit with their pharmaceutical sponsors. The CRO will assist in smooth and rapid study start up, assist in subject recruitment and make sure all site-related documentation is accurate and organized. This allows clinical research sites to spend less time on operations work and focus on patient care using cutting-edge technology and the most efficient processes.
Another responsibility of CROs should be preparation for managing clinical trials during unforeseen emergencies, such as natural disasters or global epidemics. A current example is the unprecedented impact of the current COVID-19 pandemic. A recent query of the ClinicalTrials.gov website revealed hundreds of clinical trials have been suspended as a direct result of COVID-19.14 Some of these were halted due to unavoidable reasons, such as federal mandates which have prevented in-person clinical visits; however, CROs should be prepared to switch to virtual patient visits and telemedicine whenever feasible. The success of teledermatology has already been demonstrated for increasing treatment availability for patients living in remote areas with significant cost savings.15 Satisfaction with telemedicine appears to be greatest among younger patients and those with longer travel times.16 During the COVID-19 pandemic, teledermatology was used to diagnose and manage patients with acne, atopic dermatitis, and skin-related infections,15, 17, 18 and clinical trials have successfully employed teleconsultations for treating moderate-to-severe acne.19 Thus, CROs participating in dermatology research should consider an emergency response plan when feasible in the event traditional research protocols become disrupted.
The ideal CRO should recruit and assist top dermatology and aesthetic sites that include large potential patient populations in close proximity to the study locations, leveraging existing abilities and resources providing turnkey CRO capabilities. Upon site activation, the recruitment team will immediately begin funneling potential study subjects in close geographic proximity to study sites for screening and enrollment. The CRO therefore uses their own CRAs and support staff to work side by side with principal investigators to facilitate an optimized operational model consistently across all sites. A full range of services will include subject recruiting, scientific consultation, protocol development, medical writing, project management, clinical and medical monitoring, data management, biostatistics, and pharmacovigilance.
Conclusion
CROs serve a vital role in the drug development process; however, improvements in the traditional CRO model can be made. The next generation CRO will act as a partner with researchers in dermatology and aesthetic medicine by taking advantage of the extensive experience in dermatology and aesthetics, coupled with the CRO’s proven track record for launching new drugs, the ability to move a drug through each phase of the drug development process, and experience in communicating with regulatory agencies.
Acknowledgments
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., during the preparation of this manuscript. This work was sponsored by The Vial Dermatology CRO, San Francisco, CA.
References
- Business Wire. Dermatology Drugs Market Research Report 2020 – Global Industry Analysis and Growth Forecast to 2030. March 15, 2021. Available: https://www.businesswire.com/news/home/20210315005576/en/Dermatology-Drugs-Market-Research-Report-2020—Global-Industry-Analysis-and-Growth-Forecast-to-2030—ResearchAndMarkets.com.
- Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmsted County, Minnesota, 2000 to 2010. Mayo Clin Proc. 2017;92:890–898.
- Nestor MS, Zarraga MB. The incidence of nonmelanoma skin cancers and actinic keratoses in South Florida. J Clin Aesthet Dermatol. 2012;5:20–24.
- Boen M, Jerdan K. Growing impact of social media in aesthetics: review and debate. Clin Dermatol. 2022;40:45–48.
- American Society of Plastic Surgeons. 2020 Plastic Surgery Statistics.
- Maisel A, Waldman A, Furlan K, et al. Self-reported patient motivations for seeking cosmetic procedures. JAMA Dermatol 2018;154:1167–1174.
- FDA. Good Clinical Practice. December 11, 2019. Available: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/good-clinical-practice.essed: March 12, 2022.
- Walsh R. A history of: Contract Research Organisations (CROs). Pharmaphorum, November 10, 2010. Available: https://pharmaphorum.com/views-and-analysis/a_history_of_contract_research_organisations_cros/.
- Greene JA, Podolsky SH. Reform, regulation, and pharmaceuticals–the Kefauver-Harris Amendments at 50. N Engl J Med. 2012;367:1481–1483.
- FDA Consumer Health Information. Kefauver-Harris Amendments Revolutionized Drug Development, October 2012. Available: https://www.gvsu.edu/cms4/asset/F51281F0-00AF-E25A-5BF632E8D4A243C7/kefauver-harris_amendments.fda.thalidomide.pdf. Accessed: February 14, 2022.
- Anon. Contract Research Organizations in the US – Number of Businesses 2003-2026. IBISWorld, November 28, 2020. Available: https://www.ibisworld.com/industry-statistics/number-of-businesses/contract-research-organizations-united-states/. Accessed: February 15, 2022.
- Vijayananthan A, Nawawi O. The importance of Good Clinical Practice guidelines and its role in clinical trials. Biomed Imaging Interv J 2008;4:e5.
- Dombernowsky T, Haedersdal M, Lassen U, et al. Criteria for site selection in industry-sponsored clinical trials: a survey among decision-makers in biopharmaceutical companies and clinical research organizations. Trials. 2019;20:708.
- Asaad M, Habibullah NK, Butler CE. The impact of COVID-19 on clinical trials. Ann Surg. 2020;272:e222–223.
- Marasca C, Annunziata MC, Camela E, et al. Teledermatology and inflammatory skin conditions during COVID-19 era: new perspectives and applications. J Clin Med. 2022;11:1511.
- Chang M, Lipner S. Disparities in telemedicine satisfaction among older and non-white dermatology patients: a cross-sectional study. J Drugs Dermatol. 2022;21:210–214.
- Naik PP. Rise of teledermatology in the COVID-19 era: a pan-world perspective. Digit Health. 2022;8:20552076221076671.
- Gu L, Lipner SR. Review of telemedicine for management of acne patients. J Cutan Med Surg. 2022;12034754221083978.
- Moreno-Ramírez D, Duarte-Ferreras MA, Ojeda-Vila T, et al. Telemedicine management of systemic therapy with isotretinoin of patients with moderate-to-severe acne during the COVID-19 pandemic: a longitudinal prospective feasibility study. J Am Acad Dermatol. 2022;S0190-9622:00379-6.

