Clinical and Legal Considerations in Pharmaceutical Compounding
Clinical and Legal Considerations in Pharmaceutical Compounding
J Clin Aesthet Dermatol 2023;16(8 Suppl 1):S23–S28
Leon Kircik, MD, and Daniel M. Siegel, MD
Dr. Kircik is with Icahn School of Medicine at Mount Sinai in New York, New York. Dr. Siegel is with SUNY Downstate Health Sciences University in Brooklyn, New York.
Funding: Funding for this article was provided by Verrica Pharmaceutical in West Chester, Pennsylvania.
Financial Disclosures: Dr. Kircik has been a consultant, speaker, advisory board member, and/or investigator for and received honoraria and/or grant funding from the following companies: Abbott Laboratories, Abbvie, Ablynx, Aclaris, Acambis, Allergan, Inc., Almirall, Amgen, Inc., Anacor Pharmaceuticals, Anaptys, Arcutis, Arena, Assos Pharma, Astellas Pharma US, Inc., Asubio, Bausch Health, Berlex Laboratories (Bayer HealthCare Pharmaceuticals), Biogen-Idec, Biolife, Biopelle, BMS, Boehringer-Ingleheim, Breckinridge Pharma, Cassiopea, Centocor, Inc., Cellceutix, Cipher, Coherus, Colbar, Combinatrix, Connetics Corporation, Coria, Dermavant, Dermira, Dermik Laboratories, Dow Pharmaceutical Sciences, Inc., Dr. Reddy’s Lab, Dusa , Embil Pharmaceuticals, Eli Lilly, EOS, Exeltis, Ferndale Laboratories, Inc., Foamix, Ferrer, Galderma, Genentech, Inc., GlaxoSmithKline, PLC, Glenmark, Health Point, LTD, Idera, Incyte, Intendis, Innocutis, Innovail, Isdin, Johnson & Johnson, Kyowakirin Laboratory Skin Care Inc., Leo, L’Oreal, 3M, Maruho Medical International Technologies, Merck, Medicis Pharmaceutical Corp., Merz, Nano Bio, Novartis AG, Noven Pharmaceuticals, Nucryst Pharmaceuticals Corp., Obagi, Onset, OrthoNeutrogena, PediaPharma, Pfizer, Promius, PuraCap, PharmaDerm, QLT, Inc., Quinnova, Quatrix, Regeneron, Sanofi, Serono (Merck Serono International SA), SkinMedica, Inc., Stiefel Laboratories, Inc., Sun Pharma, Taro, TolerRx, Triax, UCB, Valeant Pharmaceuticals Intl., Verrica Pharmaceutical, Warner-Chilcott, XenoPort, and/or ZAGE. Dr. Siegel has been a consultant for Verrica Pharmaceutical in West Chester, Pennsylvania; he reports no other conflicts of interest relevant to the content of this article.
ABSTRACT: It is not uncommon for dermatologists to utilize compounded drug formulations to address specific patient needs that cannot be met using commercially available drugs. Some dermatology practices may derive certain economic benefits and convenience for their patients by compounding formulations in-house. Compounded drugs are considered off-label; thus, they are not approved by the United States Food and Drug Administration (FDA). Oversight of compounding pharmacies and in-clinic compounding varies by state, although the FDA has issued guidance on compounding pharmacies and regulates outsourcing and 503(B) pharmacies. Dermatologists should be aware of the legal and regulatory issues of pharmaceutical compounding, as well as safety issues and penalties associated with compounding violations. Some controversies in compounding include the use of drug moieties that have been recalled by the FDA, compounding commercially available drugs for economic reasons (cheaper than brand names), or compounding drugs when reasonable alternatives are available commercially. This article reviews the regulatory, legal, and clinical considerations of pharmaceutical compounding in the field of dermatology.
Compounded drugs are combinations or mixtures of specific ingredients or other drugs that are prepared by a licensed pharmacist, by someone working directly under the supervision of a licensed pharmacist, or by a physician for patients who have specific medical needs that presumably cannot be adequately met by commercially available products.1,2 By definition, compounded drugs are considered off-label because they have not been approved by the United States Food and Drug Administration (FDA), as formulated, for market release for a given indication.1 Arguably, dermatologists rely on pharmaceutical compounding more than physicians in other fields of medicine;3 thus, it is imperative that dermatologists understand and carefully consider the regulatory and clinical issues involved in pharmaceutical compounding to avoid potential patient safety issues and legal consequences.
Regulatory Issues in Compounding Drugs
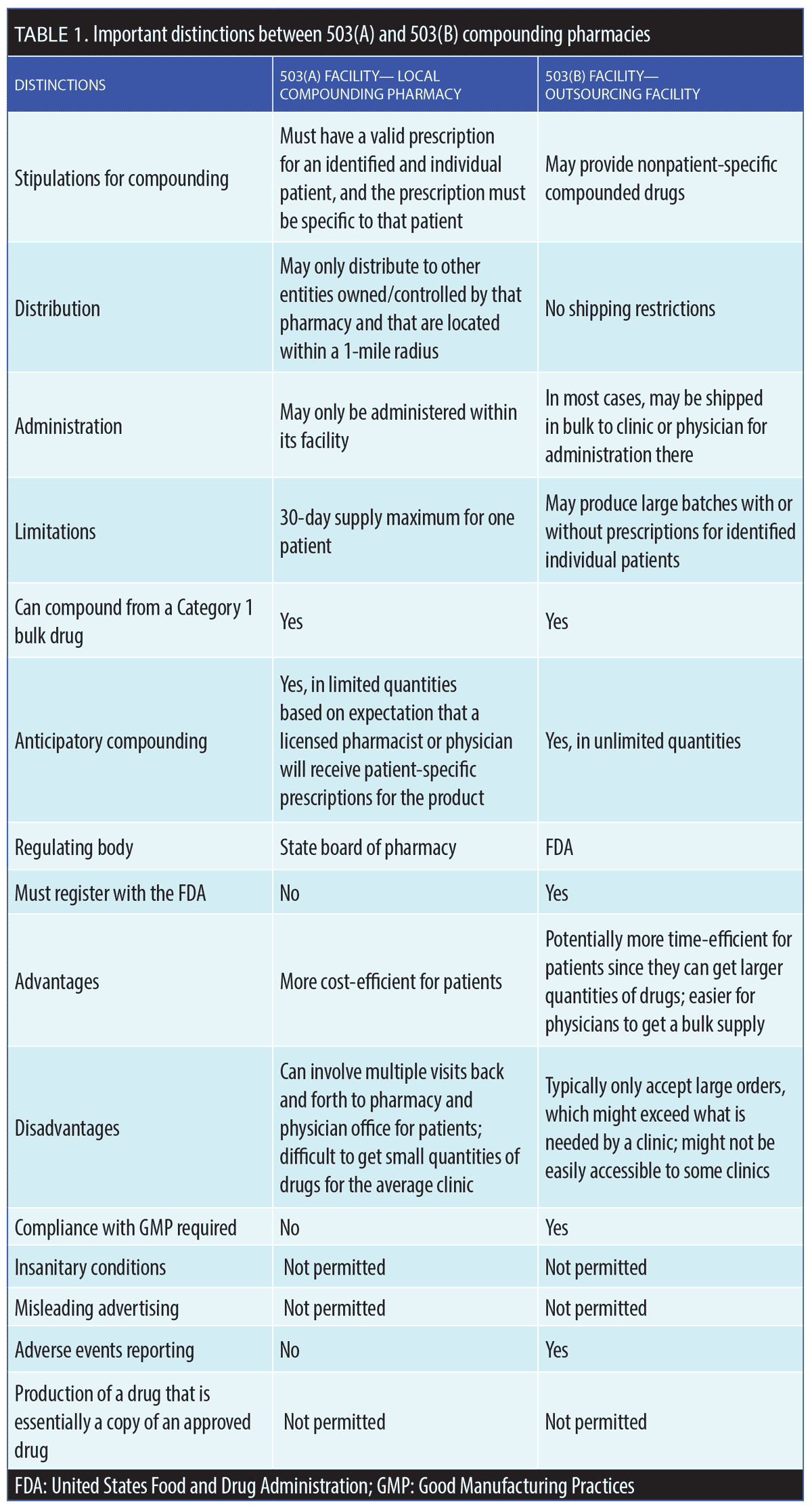
Drug Quality and Security Act (DQSA). In 2013, the Federal Food, Drug, and Cosmetic Act (FD&C Act) was amended to add the DQSA to address serious adverse events related to compounded products of poor quality.4 Under the DQSA, federally regulated compounding facilities were created, known as “outsourcing facilities” or 503(B) facilities. By and large, state boards of pharmacy have responsibility for overseeing the day-to-day activities of state-licensed compounding pharmacies or 503(A) facilities, whereas the 503(B) facilities are regulated by the FDA. A 503(B) outsourcing facility is defined as a facility in one specific geographical location (address) engaged in compounding sterile drugs in compliance with Good Manufacturing Practices (GMP) standards and all other relevant regulations.2 These two compounding designations—503(A) and 503(B)—can lead to confusion when a local compounding pharmacy starts to act as an outsourcing facility. Table 1 lists distinguishing characteristics of 503(A) and 503(B) compounding facilities. Since the purpose of compounding pharmacies is to create necessary drug formulations that are not commercially available, it is illegal to compound formulations that copy existing drugs that are currently FDA-approved for market release and are commercially available.1,3 This restriction also applies if a compounding pharmacy can make a drug similar to a commercially available formulation at a cheaper price. Additionally, is it not permissible to compound a formulation that is considered the intellectual property of another entity or when a suitable commercial product is available.1,3
The regulatory oversight of compounding pharmacies is not as stringent as it is for the preparation of FDA-approved drugs.2 The DQSA’s Compounding Quality Act designates who is responsible for the oversight of compounding pharmacies, which includes the FDA, the Centers for Medicare and Medicaid Services (CMS), the state boards of medicine, the state boards of pharmacy, the Stark Law (an antitrust law), and the US Pharmacopeial Convention (USP). The use and extent of physician-supervised compounding varies widely among medical specialties, but dermatologists, who commonly utilize compounding, may be particularly affected by any changes to compounding regulations.
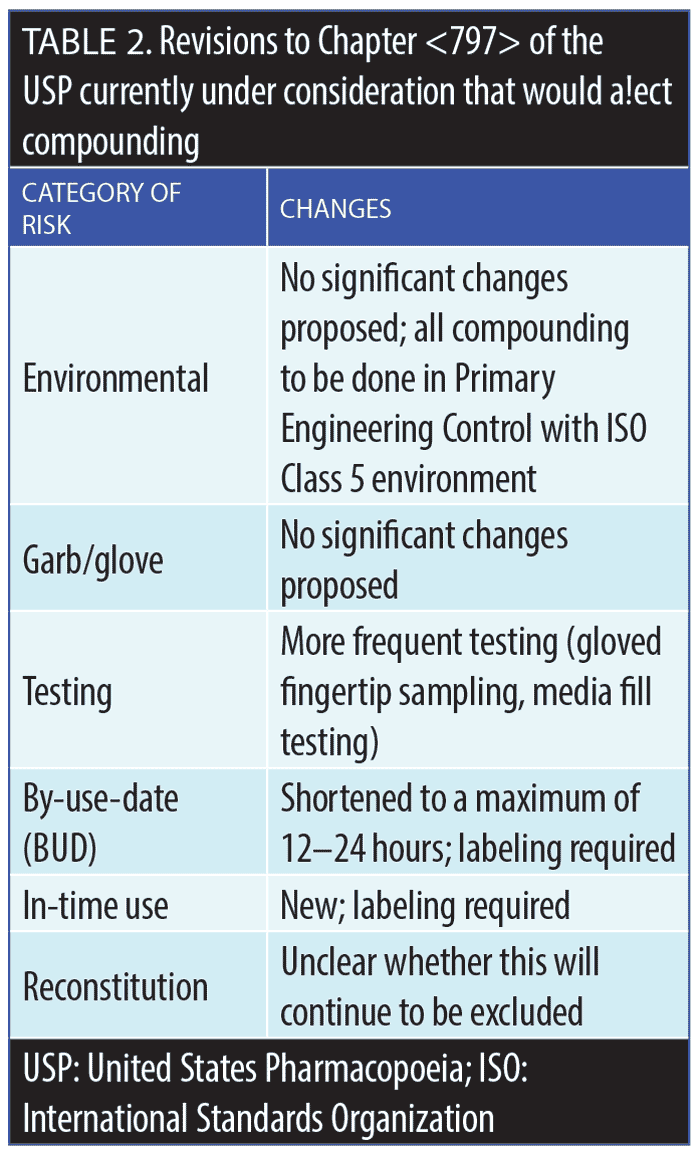
United States Pharmacopeial Convention. The USP is a private organization that predates the FDA, and it plays a major role in the US healthcare system. The main activity of the USP is the publication and subsequent updates of detailed, important information on all drug products. The FDA, which by contrast is a government organization, often relies on the USP in its recommendations and regulations, including the FDA’s lists of drug substances that are legally permissible for use in compounding, as well as those substances that have been banned for use in compounding; this list is periodically evaluated and revised.5 The USP General Chapter <797> provides detailed information about compounding medications in terms of sterility, responsibilities, safety, storage, and other requirements.5 Proposed revisions to <797> are described in Table 2. The USP guidance, unfortunately, is somewhat ambiguous on whether reconstitution or dilution of marketed products is considered compounding. For example, would it be considered compounding if an oncologist added a chemotherapy drug to an IV bag of normal saline and administered it to a cancer patient? Many would agree that this should not be considered compounding; however, the USP documentation on this matter remains unclear. Clearer definitions of what is and what is not considered compounding are needed.
Insanitary conditions at compounding facilities can have serious and potentially lethal consequences. For example, in 2012, contaminated medications produced by a compounding pharmacy in New England resulted in fungal meningitis infections in over 800 people, causing several deaths.6 The owner of the compounding center was convicted in court and sentenced to prison. The DQSA, passed in 2013, was designed to provide closer and more comprehensive oversight of compounding, and established the 503(B) outsourcing designation for compounding facilities (Table 1).7
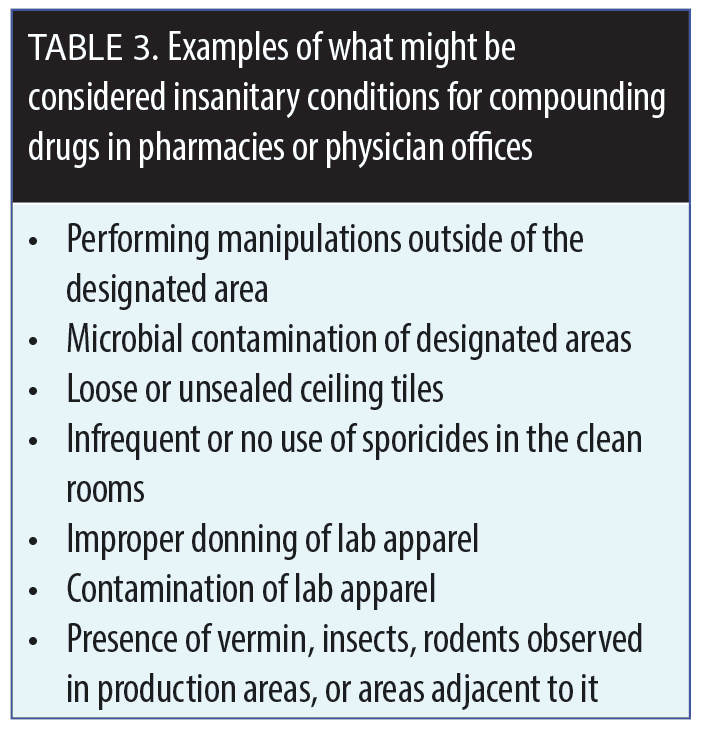
A drug is considered to be adulterated if it is prepared, packaged, or produced under “insanitary conditions.” Regulations may allow physicians to compound up to a 30-day supply of a formulation in anticipation of need based on previous requirements and for a specific patient(s), but this process requires documentation and meticulous record keeping. New draft guidance has proposed that those physicians who compound drugs in their offices use laminar flow hoods and other specialized equipment.8 Examples of insanitary conditions are listed in Table 3.
Physicians compounding in their offices are expected to follow USP standards and FDA guidelines, along with relevant state laws. It should be noted that states in the US have varying laws regarding their roles and level of oversight when it comes to in-office compounding by physicians, and while some states’ laws are stringent, others are more limited.9
Potential Legal Issues of Compounding Drugs
Physicians who prescribe compounded drugs may be held legally liable if use of a compounded product resulted in an adverse event and it could not be proven that the ingredients used in the compounded formulation were pure, sterile, free of contaminants, and/or created at the correct dose.10 Liability for adverse outcomes related to any drug not approved by the FDA can be substantial. Thus, it is good medical and legal practice for prescribers to use FDA-cleared products for patients to the fullest extent possible. If patients require products that are not commercially available, compounding may be a good option, but the prescriber should fully understand the legal and regulatory ramifications of compounding.10
Civil and criminal actions against compounding pharmacies since July 2015 expect to recover over $20 million; the primary allegations are kickbacks for prescriptions (e.g., the compounding pharmacy incentivizes doctors for prescriptions), fraudulent prescriptions (e.g., compounded products that were not necessary or that duplicate existing drugs), and violations against insurance companies or Medicare programs.11 Other charges are also possible. For example, in Minnesota, a physician was indicted for receiving kickbacks from a compounding pharmacy for topical pain cream prescriptions due to use of bulk dry ingredients that were not covered by Medicare Part D, and the compounding pharmacy was indicted for billing CMS fraudulently for the more expensive products that it did not use.9,11
Violations of these laws are not trivial offenses. A first violation for compounding a formulation that essentially copies a commercially available drug is considered criminal, with a maximum penalty of one year imprisonment, a fine up to $1,000, or both. A second violation carries a possible sentence of three years in prison, a fine up to $10,000, or both. Physicians or pharmacists who violate these rules may face exclusion from Medicare and Medicaid.12,13 Physician compounding done within the physician’s practice is subject to federal and state laws, and legal actions may include a warning letter, product(s) seizure, and/or injunction.
House Resolution 2871, the Preserving Patient Access to Compounded Medications Act, allows “office-use” compounding to be done by state-licensed pharmacies and requires the FDA to follow formal procedures for making rules and regulations. The idea behind this bill is to preserve office-based, small-volume compounding, such as is frequently used by dermatology practices.14 However, in-office compounding is still subject to following FDA regulations (mainly with respect to maintaining sanitary conditions), the USP (primarily <797> on sterility), the Federation of State Medical Board (FSMB) Sterile Compounding in Physicians’ Offices draft position system, and state boards of pharmacy. It should be noted that the FSMB was in favor of ceasing in-office drug compounding. There is, however, an “urgent use exemption” based on using a drug that was compounded in-office within 24 hours of its compounding.
Patient-specific compounding may also be carried out by 503(A) pharmacies and physician offices or clinics and in other healthcare settings. The oversight of compounding in these settings, however, needs to be better defined. A state board of medicine may, in some instances, regulate the compounding activities of an individual physician, but this varies by state. Notably, many state laws do not specifically address compounding outside of a pharmacy, although it is legal.7 Thus, while some states have specific laws in place for compounding pharmacies, others tend to regulate them only as the need arises.
Because of the complex regulations involved in compounding and the potential legal ramifications surrounding patient safety, in-office compounding may affect malpractice insurance. Should the FDA have concerns about particular compounded products, it would likely refer the matter to the appropriate state board of pharmacy.10 Dermatologists who compound products in-office or who use compounded products should be aware of the state and federal laws and review them periodically for changes.
Considerations and Potential Pitfalls of Compounding Drugs
In-office compounding. The potential benefits of in-office compounding include expansion of prescribing capabilities, greater physician control over prescribed formulations, and improved patient care. It may also serve as an income stream. However, physicians interested in compounding products in their offices should be cognizant of state regulations and their own legal liability, which can be ambiguously defined.15 While state boards of medicine regulate the practice of medicine within their respective states, this regulation does not always extend to physicians compounding within their own practices. Compounding laws vary, and many states do not have regulations specific to non-pharmacists who compound drugs. Thus, compounding on a small scale may not be worth the potential legal and regulatory issues.
Patient documentation. Prescribing a compounded drug may potentially expose a healthcare professional to legal liability should a patient have a negative outcome.10 Thus, if a prescriber feels that a patient would benefit from a medical formulation that is not commercially available, he or she should discuss the potential risks and benefits with the patient and document such discussions, including the why the compounded product is needed and why commercially available products are inadequate, as well as obtain informed consent from the patient.2 The FDA has maintained that if a compounded product offers a “significant difference” for the improved care of the individual patient compared to commercially available alternative(s), this is a valid reason to use a compounded product. The FDA does not consider cost difference between a commercially available product and a compounded copy of the product to be a valid reason to prescribe a compounded drug to a patient.16
Topical compounding. Topical products are a mainstay in dermatology and fall under the same compounding regulations as injectable drugs. Dermatologists may have medications compounded that contain ingredients that should be used only under direct clinical supervision (e.g., lidocaine, benzocaine, cantharidin). However, compounding pharmacies in the 503(A) category require such compounded medications to be specific to a patient, and it may not be safe to have a patient take such compounded products home for self-administration. In some cases, a 503(B) compounding pharmacy can make larger quantities of a topical formulation that is not specific to an individual patient, but these outsourcing pharmacies typically only take large orders, which may exceed what a typical practice actually needs. Additionally, not all dermatology practices have easy access to 503(B) compounding pharmacies.17
Packaging and labeling. Commercial products are appropriately packaged, clearly labeled, and have good shelf stability; they may last longer than an inventory of raw materials and excipients.18 Since compounded drugs do not carry the same standardized labeling or instructions for use as FDA-approved drugs,2 dermatologists should provide this information to their patients when prescribing compounded medications and carefully document such discussions.
Adverse events. Except for 503(B) facilities, compounding pharmacies are not required to report adverse events to the FDA, though this is a requirement for manufacturers of approved drugs. For that reason, the safety profiles of many compounded formulations remain unknown, particularly if they are made by many different compounding pharmacies at different locations. Morbidity and mortality data of many compounded products are not reported and thus remain unknown. Additionally, with the exception of possible reporting by the FDA or the US Centers for Disease Control and Prevention (CDC), sentinel events are not reported by compounding pharmacies.10
Compounding economics. In 2006, Medicare Part D spent about $70 million on compounded drugs, which rose to $508.7 billion by 2015, a 625-percent increase, outpacing the growth in Part D spending on other drugs (Figure 1).12 The concern with this rapid escalation is that the compounded drugs may not have been medically necessary or appropriately dispensed, topical products in particular.12 Additionally, the number of beneficiaries who were dispensed such compounded products increased 281 percent from 2006 to 2015.12 This suggests the need for continuing scrutiny on the practice of drug compounding for the foreseeable future.
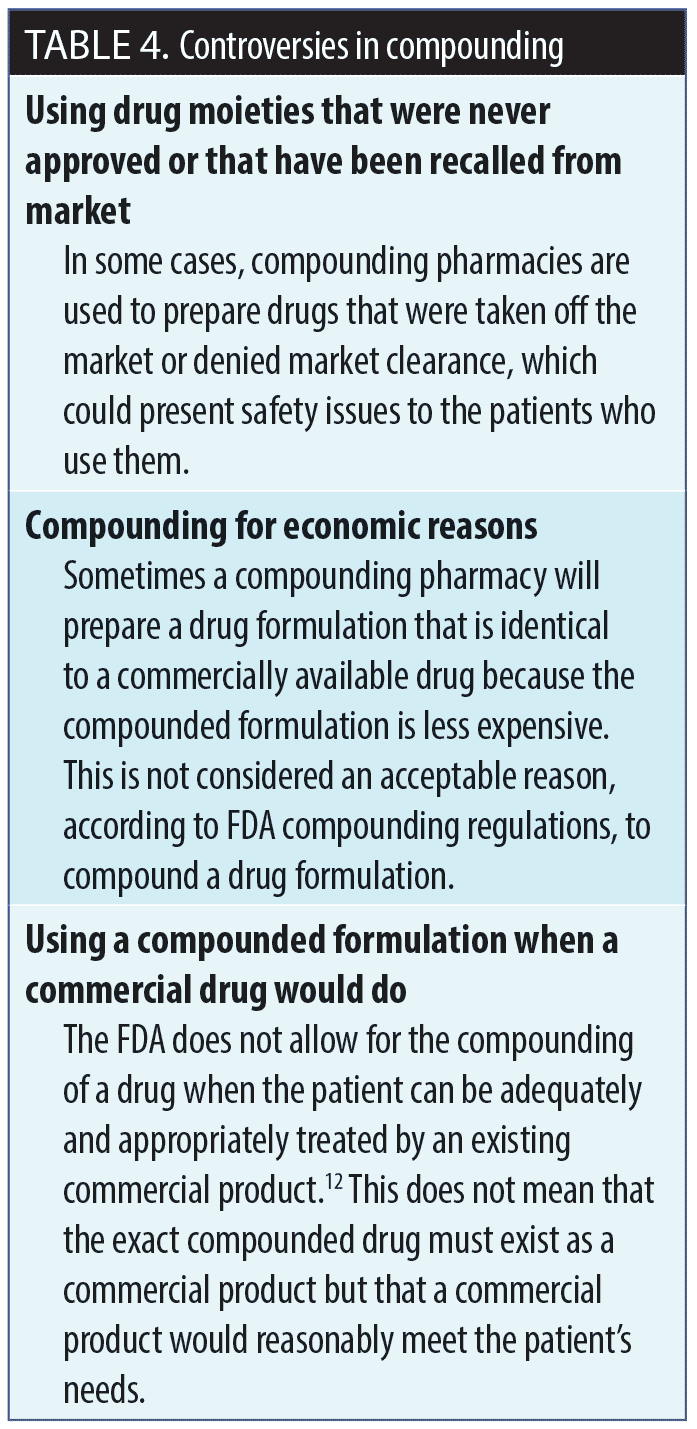
Making the decision to compound. Before moving forward with compounding a drug, there are a number of factors that should be taken into consideration: 1) Is there an available commercial product that would meet the needs being addressed by a compounded formulation? 2) Are all of the ingredients in the compounded formulation FDA-approved? 3) Is the facility that is doing the compounding registered with the FDA and fully compliant with its guidelines? A compounding decision algorithm is provided in Figure 2, and controversies surrounding compounding are summarized in Table 4.
Conclusion
The need for guidelines to assure the safety and quality of compounded drug formulations is evident. Arguably, dermatologists do more compounding in their clinics than other medical specialists. Such compounding is legal with a prescription for an individual and identified patient. New regulations for compounding pharmacies have caused some confusion regarding in-office small-scale compounding. Compounding pharmacies have now been divided into 503(A) and 503(B) facilities, with the latter being larger and under FDA regulation. Since laws are complex and consequences for medical, pharmaceutical, or legal missteps can be severe, dermatologists may wish to rely on commercially available, FDA-approved products or compounding pharmacies to meet the special needs of their patients, rather than on in-office compounding, even on a limited basis. A sound risk management strategy for a dermatology clinic should include careful consideration of the risks and benefits of compounding, such as appropriate uses and whether it could be done in-office in full compliance of state and federal laws and regulations.
Acknowledgments
The authors wish to acknowledge LeQ Medical in Angleton, Texas, for their support in preparing this manuscript.
References
- United States Food and Drug Administration site. Compounding and the FDA: questions and answers. Last updated 21 Jun 2018. https://www.fda.gov/drugs/human-drug compounding/compounding-and-fda-questions-and-answers. Accessed 26 Apr 2021.
- Gudeman J, Jozwiakowski M, Chollet J, Randell M. Potential risks of pharmacy compounding. Drugs R D. 2013;13(1):1–8.
- Kircik LH. Yes to appropriate compounding, no to illegal compounding. J Drugs Dermatol. 2018;17(7):s15–s16.
- US Congress site. H.R. 3204-Drug Quality and Security Act. https://www.congress.gov/bill/113th-congress/house-bill/3204. Accessed 26 Apr 2021.
- Agnetta V, Torres A, Desai S, et al. Compounding in dermatology update. J Drugs Dermatol. 2020;19(2):S18–S23.
- United States Food and Drug Administration site. Owner of New England compounding center sentenced for racketeering leading to nationwide fungal meningitis outbreak. Last updated 27 Mar 2017. Food and Drug Administration Office of Criminal Investigations. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/press-releases/march-22-2017-owner-new-england-compounding-center-convicted-racketeering-leading-nationwide-fungal. Accessed 14 Jun 2021
- Quertermous J, Desai S, Harper J, et al. The practice of compounding, associated compounding regulations, and the impact on dermatologists. J Drugs Dermatol. 2018;17(7):s17–s22.
- United States Food and Drug Administration site. Insanitary conditions at compounding facilities-guidance for industry. Lasted updated 6 Nov 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/insanitary-conditions-compounding-facilities-guidance-industry. Accessed 5 May 2021.
- United States Federal Bureau of Investigation site. Bloomington pain management doctor indicted for accepting kickback as part of large-scale health care fraud scheme. US Attorney’s Office. 23 Sep 2015. https://www.fbi.gov/contact-us/field-offices/minneapolis/news/press-releases/bloomington-pain-management-doctor-indicted-for-accepting-kickbacks-as-part-of-large-scale-health-care-fraud-scheme. Accessed 5 May 2021.
- Sellers S, Utian WH. Pharmacy compounding primer for physicians: prescriber beware. Drugs. 2012;72(16):2043–2050.
- United States Food and Drug Administration site. Compounding laws and policies. Human drug compounding. Published 2020. https://www.fda.gov/drugs/human-drug-compounding/compounding-laws-and-policies. Accessed 30 Aug 2021.
- United States Office of the Inspector General. High Part D spending on opioids and substantial growth in compounded drugs raise concerns. United States Department of Health and Human Services. HHS OIG Data Brief. June 2016. OEI-02-16-00290. https://oig.hhs.gov/oei/reports/oei-02-16-00290.pdf. Accessed 30 Aug 2021.
- Souze C. The penalties under DSCSA. TrackTraceRx site. https://blog.tracktracerx.com/the-penalties-under-dscsa. Accessed 11 May 2021.
- United States Congress site. HR 2871–Preserving Patient Access to Compounded Medications Act of 2017. https://www.congress.gov/bill/115th-congress/house-bill/2871. Accessed 5 May 2021.
- Pergolizzi JV, Labhsetwar S, LeQuang JA. Compounding pharmacies: who is in charge? Pain Pract. 2013;13(3):253–257.
- United States Food and Drug Administration site. Compounded drug products that are essentially copies of a commercially available drug product under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry. Published 2018. https://www.fda.gov/media/98973/download. Accessed 4 May 2021.
- Kircik L, Torres A, Desai S, Hebert A. Compounding dermatology update-part 2: next steps in dermatology. 21 Mar 2021. Journal of Drugs in Dermatology site. https://nextstepsinderm.com/derm-topics/compounding-in-dermatology-update-part-2/. Accessed 5 May 2021.
- Kircik L. To compound or not to compound. J Drugs Dermatol. 2020;19(2):S16–S17.