 J Clin Aesthet Dermatol. 2020;13(3):37–40
J Clin Aesthet Dermatol. 2020;13(3):37–40
by Sherine Hosny Abdel Rahman, MD; Rehab Mohammed Salem, MD; and Jehan Hassan Sabry, MD
All authors are with the Faculty of Medicine at Benha University in Benha, Egypt.
FUNDING: No funding was provided for this study.
DISCLOSURES: The authors have no conflicts of interest relevant to the content of this article.
ABSTRACT: Background. Biotin is popular in hair loss treatment supplements, although the frequency of its deficiency in patients with hair loss has not been established.
Objectives. This work sought to assess the serum levels of biotin in patients with telogen effluvium.
Methods. This case-control study included 60 patients with telogen effluvium and 20 control subjects. Subjects who were on biotin therapy three months prior to the study and those reporting other causes of hair loss were excluded. The scalp of each patient was clinically and dermoscopically examined. Serum biotin levels were measured using enzyme-linked immunosorbent assay kits.
Results. Serum levels of biotin were optimal in patients and control groups with no significant difference between the groups. Insignificantly lower biotin levels in elderly patients, smokers, athletes, those with a history of recurrent infections, and women who were pregnant and/or lactating were observed. There was also an insignificant positive correlation between the serum level of biotin and patient age and an insignificant negative correlation between disease duration and patient body mass index. Serum biotin has a weak specificity and sensitivity in differentiating between cases and control subjects or between acute and chronic telogen effluvium.
Conclusion. There was no significant difference in serum biotin levels between cases and controls or between those with acute or chronic telogen effluvium.
KEYWORDS: Biotin, hair loss, nutritional deficiency, telogen effluvium, vitamin B7
Telogen effluvium (TE) is a form of nonscarring alopecia characterized by diffuse hair shedding, often with an acute onset. A chronic form with a more insidious onset and a longer duration also exists.1,2 TE is an abnormality in the hair cycle that occurs as a reaction pattern to various physical or mental stressors. The degree of effluvium depends on the severity and duration of exposure rather than the type of stressor.3
The quantity and quality of an individual’s hair are closely related to his or her nutritional state. Normal supply, uptake, and transport of proteins, calories, trace elements, and vitamins are of fundamental importance in tissues with a high biosynthetic activity, such as the hair follicle.4 Currently, there are many commercially available nutritional supplements that claim to treat hair problems; however, scientifically, it is still unclear whether supplementing the hair follicle with a nutrient in which the body is not deficient will enhance hair growth.
Due to its availability, affordability, and effectiveness, biotin is a popular nutritional supplement for the treatment of brittle nails and hair loss. There are no known toxicity or adverse effects of receiving high doses of biotin.5 While the response of splitting, brittle nails (i.e., onychoschizia, onychoschisis) to daily biotin supplementation, irrespective of serum biotin levels, has been demonstrated,6 little data exists on the frequency of biotin deficiency in the general population and in patients reporting hair loss.5 Currently, there is no available information on the relationship between hair loss and abnormal biotin levels.7
Biotin deficiency can affect the skin, immune system, and nervous system.8 The dermatologic manifestations of its deficiency include hair loss and scaly, erythematous, eczematous periorificial lesions resembling those characteristic of zinc deficiency.9 Regarding the skin findings of biotin deficiency, the role of deficient biotin in the development of seborrheic dermatitis and the role of its supplementation have been suggested.10 However, biotin’s role in hair disorders and its efficacy in treating them remains an area of debate. Rauch11 suggested that biotin might be related to normal development, maintenance, and pigmentation of hair. Later, Georgala et al12 proposed the importance of biotinidase activity evaluation in patients with alopecia areata, due to the significantly lower activity of biotin in these patients and the improvement seen in the patients following biotin supplementation.
The current work aims to assess the serum levels of biotin and to determine the frequency of its deficiency in a group of patients with TE.
Materials and Methods
This case-control study was conducted at the outpatient clinic of the Dermatology and Andrology Department at Benha University to evaluate the serum levels of biotin in patients with TE. The study included 60 patients with TE and 20 healthy subjects matched for age, sex, and body mass index (BMI). This study was approved by the Hospital Ethics Committee on research involving human subjects in the Faculty of Medicine. Written informed consent was obtained from every participant before sample collection. Subjects who were on biotin therapy three months prior to the study and those reporting other causes of hair loss, such as androgenetic alopecia, were excluded from this study. Patients were subjected to full history-taking, including onset, course, and duration of hair loss, stressful conditions, and history of prescriptions and any other medical conditions. The scalp was examined carefully to exclude patients with concomitant seborrheic dermatitis or patients with other causes of hair loss. A pull test was performed and dermoscopic examination of the scalp was carried out using a dermoscope (Dermlite DL4; 3Gen LLC, San Juan Capistrano, California).
Five milliliters of venous blood was withdrawn from each subject into a plain serum-separating tube and allowed to clot for 30 minutes. Next, the blood was centrifuged at 3,000rpm for 10 minutes, and sera were separated and stored at -20 degrees C for further tests. Biotin serum levels were measured using a commercially available biotin enzyme-linked immunosorbent assay kit (Manual IDK® Biotin ELISA; Immundiagnostik, Bensheim, Germany).
Statistical analysis. All statistical analyses were carried out in STATA/SE version 11.2 for Windows (StataCorp LLC, College Station, Texas) and p-values of less than 0.05 were considered to be statistically significant.
Results
This case-control study was conducted on 60 patients with TE and 20 healthy subjects. Patients and control subjects were matched in terms of age (30.65±8.07 years vs. 31.0±8.18 years, respectively; p=0.87) and sex (88.3% vs. 80% female patients, respectively; p=0.45).
In the patient group, family history of telogen effluvium was positive among 50 percent of the patients. Additionally, 6.7 percent were smokers, 13.3 percent were athletes, and 6.7 percent had recurrent infections. Twenty percent of the patients were pregnant and 30 percent had psychological symptoms, including decreased self esteem, depression, and distress. None of the patients were undergoing prolonged antibiotic treatment or taking concomitant isotretinoin or antiepileptics.
The onset of the disease was sudden in 51.7 percent of subjects and the course of the disease was chronic among 66.7 percent. The mean duration of the disease was 10.5±8.75 years. Clinical and trichoscopic examination findings are shown in Table 1.

Although serum levels of biotin were optimal (>0.4ng/mL) in both groups, control subjects showed higher serum levels of biotin, with no significant difference (p=0.59) between the two groups (5.38±4.01 vs. 4.83±3.94ng/mL, respectively). Although there was no significant difference in the serum levels of biotin in relation to different demographic criteria and history findings, relatively lower levels were recorded in elderly patients, smokers, athletes, those who had a history of recurrent infections, and women who were pregnant and/or lactating (Table 2).

Serum levels in patients with gradual onset of TE were higher than those with sudden onset (5.66±4.84 vs. 4.05±2.71 ng/mL respectively) and serum levels in chronic TE were higher than those with acute TE (5.06±4.49 vs. 4.36±2.54ng/mL); however, the difference was not significant (p=0.32 and p=0.40, respectively). There was an insignificant positive correlation between serum levels of biotin and the age of the patients (r=0.004; p=0.99), and an insignificant negative correlation with the disease duration (r=-0.11; p=0.42) and BMI (r=-0.12; p=0.37).
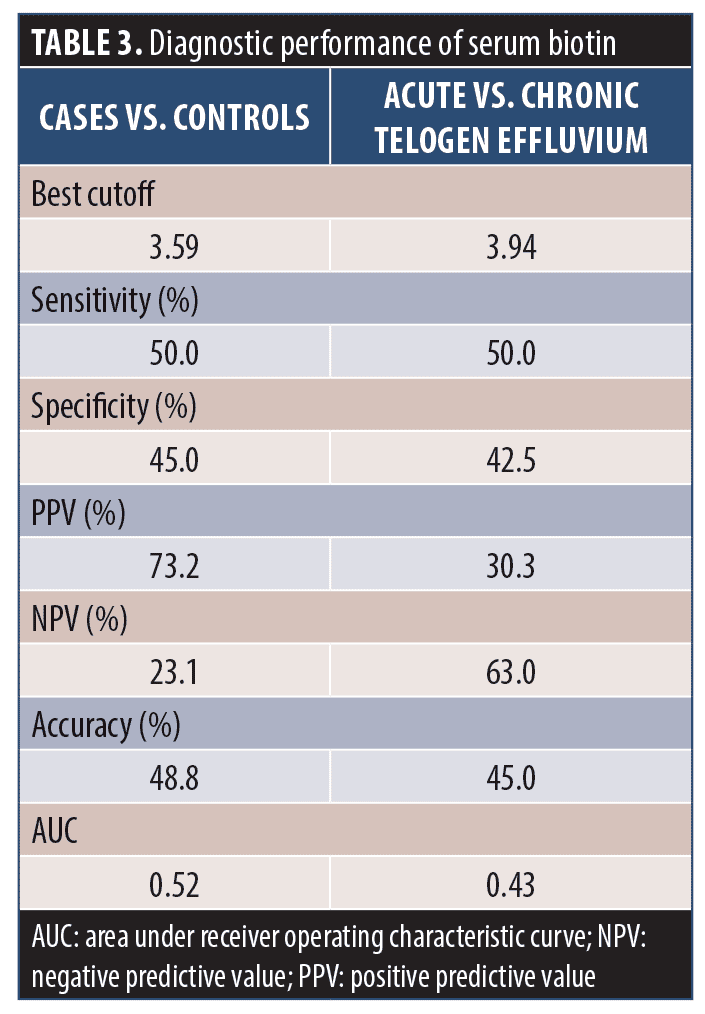
Receiver operating characteristic curve analysis of the diagnostic performance of biotin serum levels showed that serum biotin had weak specificity and sensitivity in differentiating between cases and control subjects or between acute and chronic TE (Table 3).
Discussion
Biotin (vitamin B7 or vitamin H) is a member of the vitamin B complex. It is a water-soluble vitamin that is essential for a range of metabolic reactions, due to its role as an important cofactor for carboxylase enzymes in humans.13 Optimal serum biotin levels are more than 400ng/L, while serum levels of between 200 to 400ng/L are considered suboptimal. Biotin is considered deficient when its levels are less than 100ng/L.4 Our results indicated optimal serum levels of biotin in all participants. This finding was observed in another study,10 which described biotin deficiency as rare, due to its production by normal intestinal flora and ready availability in many diets. Dobrowolski et al14 found that there are some inherited causes of biotin deficiency, such as the biotinidase deficiency, which is an autosomal-recessive trait. Other studies have also reported some acquired conditions in which there are relatively low levels of biotin, such as parenteral nutrition or achlorhydria or in patients on anticonvulsants or isotretinoin.15–18
Our investigation did not reveal any significant difference in serum levels of biotin between the patients and control subjects. Moreover, the serum levels of biotin in both groups were optimal. In our study, elderly patients, smokers, athletes, those who had a history of recurrent infections, and women who were pregnant and/or lactating showed relatively lower levels of biotin among those with TE. This was in accordance with other research that asserted that smoking can accelerate biotin catabolism,19 while pregnancy20 and lactation have been associated with an increased demand for biotin. Trüeb4 reported lower levels of biotin were observed in elderly people and in those taking antibiotics for recurrent infections (which are thought to interfere with biotin metabolism). Although these factors did not seem to affect biotin serum levels significantly in our study, asking about these factors, especially in pregnant or lactating women who also smoke, would be prudent since these factors can accelerate biotin catabolism and possibly predispose these patients to marginal biotin deficiency. However, we agree with Trüeb4 that the history of the patient is minimally sensitive, due to the possibility that many patients can have one or more factors that can lead to biotin deficiency while their serum levels remain optimal.
In our study, serum levels in patients with gradual onset of hair loss were higher than those with sudden onset and serum levels in patients with chronic TE were higher than those with acute TE; however, the difference was not significant. Receiver operating characteristic curve analysis of the diagnostic performance of biotin serum levels showed that serum biotin has weak specificity and sensitivity in differentiating between cases and control subjects or between acute and chronic TE. However, serum concentrations of biotin and its catabolites are not good indicators of marginal biotin deficiency because they do not decrease sufficiently in people with marginal biotin deficiency.21
The documented effects of age and sex on serum levels of biotin are controversial.22,23 Biotin levels showed no significant relation with age, sex, or BMI in the our study. Sone et al24 elucidated that biotin suppresses the appetite by upregulation of acetyl coenzyme A carboxylase gene expression in the hypothalamus. This hypothesis might explain the negative correlation between biotin levels and BMI in the current study. The results of our study, which support Trüeb’s4 findings, would exclude the strong association between biotin deficiency and TE development. Moreover, both studies raise many questions about using biotin supplementation indiscriminately for any case of hair loss.
Biotin supplementation is considered safe, and the Scientific Committee on Food25 concluded that the usual dietary intake of biotin and biotin supplements is tolerable and the incidence of toxicity from biotin is considered low. However, given the available studies, the routine use of biotin in patients experiencing hair loss, regardless of the serum biotin level, should be discouraged and limited to cases of true biotin deficiency, especially considering the Food and Drug Administration warned about the risk of an effect on the sensitivity of certain blood tests (e.g., levels of troponin and thyroid-stimulating hormone) prompted by exogenous biotin.26,27 In many laboratory tests, biotin binding with streptavidin increases the test sensitivity; the supplementary biotin in the blood might compete with biotin in the immunoassays, affecting the reliability of the results of these tests.28 Moreover, Katzman et al29 reported that 7.4 percent of patients in an emergency department were taking biotin supplements without confirmed deficiency.
Using biotin supplements in biotin-deficient patients with brittle nails, uncombable hair, or hair loss has demonstrated great clinical improvement;5 however, the value of biotin supplementations in healthy individuals is not supported. Our study is limited by the small sample size of evaluated patients.
Conclusion
According to our study, biotin deficiency is rare and its serum levels showed no significant difference between cases and control subjects nor between acute or chronic TE. This study also stands against prescribing biotin supplements to all cases of hair loss without a confirmed biotin deficiency, as it might expose certain people to potential harm. From the previous findings, studies are needed to evaluate the effects of biotin supplements on hair loss in patients with normal levels of biotin in order to determine whether they are beneficial in this population.
References
- Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venereol Leprol. 2013;79(5):591–603.
- Torres F, Tosti A. Female pattern alopecia and telogen effluvium: figuring out diffuse alopecia. Semin Cutan Med Surg. 2015;34(2):67–71.
- Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015; 9(9):WE01–WE03.
- Trüeb RM. Serum biotin levels in women complaining of hair loss. Int J Trichology. 2016;8(2):73–77.
- Patel DP, Swink SM, Castelo-Soccio L. A review of the use of biotin for hair loss. Skin Appendage Disord. 2017;3(3):166–169.
- Cashman MW, Sloan SB. Nutrition and nail disease. Clin Dermatol. 2010;28:420–425.
- Ruiz-Tagle SA, Figueira MM, Vial V, et al. Micronutrients in hair loss. Our Dermatology Online. 2018; 9(3):320–328.
- Seymons K, De Moor A, De Raeve H, Lambert J. Dermatologic signs of biotin deficiency leading to the diagnosis of multiple carboxylase deficiency. Pediatr Dermatol. 2004;21(3):231–235.
- Mock DM. Skin manifestations of biotin deficiency. Semin Dermatol. 1991;10(4):296–302.
- Messaritakis J, Kattamis C, Karabula C, Matsaniotis N. Generalized seborrhoeic dermatitis. Clinical and therapeutic data of 25 patients. Arch Dis Child. 1975;50(11):871–874.
- Rauch H. The effects of biotin deficiency on hair development and pigmentation. Physiol Zool. 1952;25(2):145–149.
- Georgala S, Schulpis K, Papakonstantinou ED, et al. Possible involvement of partial biotinidase deficiency in alopecia areata. J Eur Acad Dermatol Venereol. 1996;7(2);135–138.
- Said HM. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem. 2012;56:1–19.
- Dobrowolski SF, Angeletti J, Banas RA, Naylor EW. Real time PCR assays to detect common mutations in the biotinidase gene and application of mutational analysis to newborn screening for biotinidase deficiency. Mol Genet Metab. 2003;78(2):100–107.
- Mock DM, Baswell DL, Baker H, et al. Biotin deficiency complicating parenteral alimentation: Diagnosis, metabolic repercussions, and treatment. J Pediatr. 1985;106(5):762–769.
- Greenway FL, Ingram DK, Ravussin E, et al. Loss of taste responds to high-dose biotin treatment. J Am Coll Nutr. 2011;30(3):178–181.
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42(10): 1359–1362.
- Schulpis KH, Georgala S, Papakonstantinou ED, et al. The effect of isotretinoin on biotinidase activity. Skin Pharmacol Appl Skin Physiol. 1999;12(1–2):28–33.
- Sealey WM, Teague AM, Stratton SL, Mock DM. Smoking accelerates biotin catabolism in women. Am J Clin Nutr. 2004;80(4):932–935.
- Mock DM, Quirk JG, Mock NI. Marginal biotin deficiency during normal pregnancy. Am J Clin Nutr. 2002;75(2):295–299.
- Zempleni J, Wijeratne SSK, Kuroishi T. Biotin. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012: 359–374.
- Watanabe T, Yasumura S, Shibata H, Fukui T. Biotin status and its correlation with other biochemical parameters in the elderly people of Japan. J Am Coll Nutr. 1998;17(1):48–53.
- Combs Jr, Gerald F. The Vitamins: Fundamental Aspects in Nutrition and Health. San Diego, CA: Elsevier, Inc.; 2008.
- Sone H, Kamiyama S, Higuchi M, et al. Biotin augments acetyl CoA carboxylase 2 gene expression in the hypothalamus, leading to the suppression of food intake in mice. Biochem Biophys Res Commun. 2016;476(3):134–139.
- European Food Safety Authority. Tolerable upper intake levels for vitamins and minerals. Available at: http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf. Accessed November 20, 2018.
- United States Food and Drug Administration. The FDA Warns that Biotin May Interfere with Lab Tests: FDA Safety Communication. Available at: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm586505.htm. Accessed November 25, 2018.
- Lam L, Bagg W, Smith G, et al. Apparent hyperthyroidism caused by biotin-like interference from IgM anti-streptavidin antibodies. Thyroid. 2018;28(8):1063–1067.
- Piketty ML, Polak M, Flechtner I, et al. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clin Chem Lab Med. 2017;55(6):780–788.
- Katzman BM, Lueke AJ, Donato LJ, et al. Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin Biochem. 2018;60:11–16.

