 J Clin Aesthet Dermatol. 2023;16(4):53–59.
J Clin Aesthet Dermatol. 2023;16(4):53–59.
by Zoe Diana Draelos, MD; David H. McDaniel, MD; Steve Yoelin, MD; Sera Pot, BS; Olivia Sotir, BS;and Diane B. Nelson, RN, MPH
Dr. Draelos is with Dermatology Consulting Services, PLLC in High Point, North Carolina. Dr. McDaniel is with McDaniel Institute of Aging Research in Virginia Beach, Virginia. Dr. Yoelin and Ms. Pot and Sotir are with Steve Yoelin, MD Medical Associates in Newport Beach, California. Ms. Nelson is with skinbetter science, LLC in Phoenix, Arizona.
FUNDING: This research was supported by skinbetter science, LLC.
DISCLOSURES: Drs. Draelos, McDaniel, and Yoelin received funds from skinbetter science, LLC, to conduct the research presented in this manuscript. Dr. McDaniel is a consultant for skinbetter science, LLC. Ms. Pot and Sotir are employees of Steve Yoelin, MD Medical Associates. Ms. Nelson is an employee of skinbetter science, LLC.
ABSTRACT: Objectives. Evaluate the effects of a new antioxidant containing topical allyl pyrroloquinoline quinone (TAP) on expression of key markers and assess the efficacy and tolerability in subjects with photodamaged skin.
Methods. Donor skin tissue was irradiated prior to and following application of study products (TAP; a leading antioxidant cream [L-VC]). Expression of markers related to epidermal homeostasis and oxidative stress were assessed at 48 hours and compared to untreated, irradiated control (n=3 each). Evaluation of lines/wrinkles, skin texture, skin tone, dullness, and erythema from baseline occurred over 12 weeks in subjects with mild-to-moderate photodamaged skin. Histological evaluation occurred at Weeks 6 and 12 (n=4).
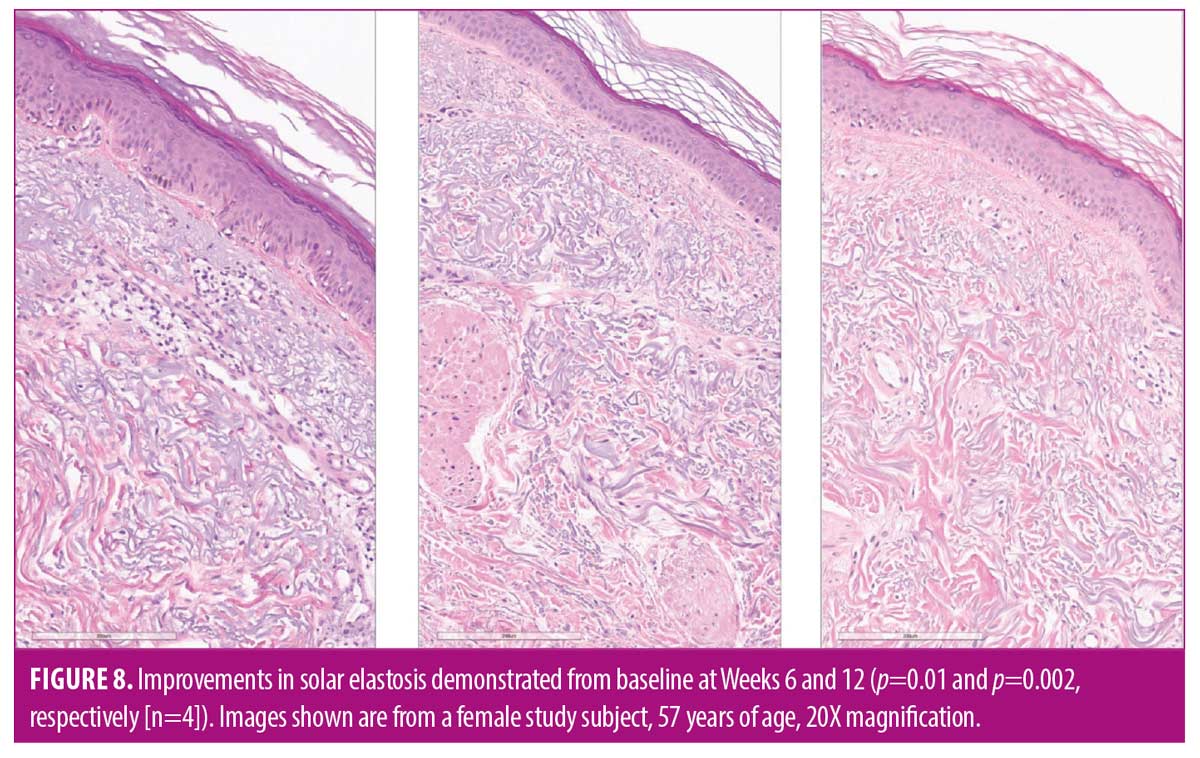
Results. Following application of TAP, significant expression of markers related to epidermal homeostasis and repair, recycling and removal, and oxidative stress were demonstrated, compared to control (p<0.05). Reduced expression of collagen degrading enzymes, compared to control, were observed (p<0.05). Application of L-VC demonstrated nonsignificant expression of markers versus control. In 40 subjects evaluated over 12 weeks, significant mean improvements from baseline were observed at Week 4 in skin texture and dullness (both p<0.0001) and skin tone and lines/wrinkles (both p=0.01). The study product was highly tolerable. Histologic evaluation demonstrated reductions in solar elastosis from baseline at Weeks 6 (33%, p=0.01) and 12 (60%, p=0.002).
Conclusion. An antioxidant containing TAP addresses internal and external manifestations of photoaging. TAP demonstrated significant expression of key markers associated with epidermal homeostasis and counteracting oxidative stress. Significant, early improvements in the appearance of photodamaged skin and histological improvements in solar elastosis were observed.
Keywords: Skin aging, intrinsic aging, mitochondrial damage, allyl PQQ, topical antioxidants
Oxidative stress is one of the core mechanisms that mediates skin aging,1 with both intrinsic and extrinsic factors contributing to the skin aging process. Environmental stressors, including ultraviolet (UV) light, infrared radiation, ozone, and tobacco trigger the generation of reactive oxygen species (ROS), causing damage to skin cells over time.2 Intrinsic aging involves a gradual reduction in the efficiency of mitochondria and acceleration of mitochondrial damage and dysfunction in skin cells. This leads to increased generation of ROS and accumulation of cellular debris. The primary function of mitochondria is generation of energy via oxidative phosphorylation. Intrinsically, mitochondria are the primary cellular source of ROS generation,3 which facilitates epidermal differentiation.4
Mitochondria are integral to skin for cell signaling, pigmentation, and vasculature homeostasis.5 The “free radical theory of aging,” which has not been fully corroborated, suggests there is a correlation between aging, mitochondrial deoxyribonucleic acid (mtDNA) damage, particularly mtDNA deletions, and increasing oxidative stress.5,6 In fact, mitochondrial dysfunction is believed to be one of the hallmarks that contributes to the pathophysiology of age-related disorders.7
Skin is naturally equipped to protect and renew itself. However, when internal defenses are overwhelmed (as a result of oxidative stress), skin is unable to repair the cumulative effects of ROS damage and thus loses the ability to function efficiently.4–12 Effects include reduced collagen and elastin production, decreased cellular regeneration and desquamation, and gradual dermal atrophy.13,14 Additionally, a reduction in the proliferation of basal cells occurs, leading to epidermal thinning.7 Intrinsic skin aging is also characterized by reduced levels of lipids in the stratum corneum.7,10
Protecting skin from free radical damage has predominantly focused on addressing the effects of extrinsic aging through the use of broad-spectrum sunscreens and antioxidants. Sunscreens scatter or block UV radiation and absorb UV rays prior to the formation of free radicals in the skin.15 In contrast, antioxidants stabilize or deactivate free radicals before they damage cells.16–18 As mitochondria are the main source of intrinsic ROS,3 mitochondrial-targeted antioxidants are essential in providing comprehensive support to skin.
Pyrroloquinoline quinone (PQQ) is a potent antioxidant/micronutrient found in a variety of foods, including spinach, green pepper, kiwi, parsley, carrots, and fava beans, as well as breast milk.19 PQQ is an efficient free radical scavenger of superoxide and hydroxyl radicals and a highly effective quinone or enediol that has traditionally been delivered orally as a dietary supplement. PQQ neutralizes ROS generated by mitochondria and has been shown to protect living cells from oxidative damage in mitochondria in-vivo.20 Specifically, PQQ helps support cellular growth and differentiation and has been shown to stimulate sirtuin, supporting DNA repair and mitochondrial biogenesis.20–22
Historically, PQQ has been challenging to formulate as a topical agent, owing to its inherent irritation and instability. Adding an allyl (ester) group to the parent molecule was shown to enhance stability and mitigate skin irritation. Allyl PQQ is a new molecule designed to deliver the benefits of PQQ directly to the skin to defend against internal stressors, broadening its antioxidant defense against free radical cellular damage. Allyl PQQ supports more efficient mitochondrial energy creation that is critical to the renewal and repair process of the skin.21
Topical allyl PQQ (TAP) is combined with existing WEL antioxidant technology (skinbetter science; Phoenix, Arizona, United States [US]) to comprehensively address both extrinsic and intrinsic aging. WEL technology is comprised of an optimized, balanced ratio of 19 potent water-soluble, enzymatic, and lipid-soluble antioxidants purposefully identified and selected to provide synergistic cellular protection against a broad range of free radicals. Previous studies performed with WEL technology evaluated its capacity to neutralize ROS (hydrogen peroxide); its protective effects against UVA/B, ozone, blue light and tobacco-induced oxidative stress; and its visible effects on photodamaged skin.2,11,23 Together, these studies provided substantial evidence demonstrating WEL technology’s ability to protect skin against oxidative stress and improve the appearance of photodamaged skin. This new, advanced formulation combines both antioxidant technologies (TAP and WEL) and additional ingredients to support skin barrier and repair to comprehensively address both intrinsic and extrinsic skin aging (Table 1).
Herein, we describe two studies evaluating the mechanistic and visible effects of this new formulation. An ex-vivo study conducted utilizing human skin tissue evaluated the effects of TAP and a leading vitamin C-based cream (L-VC) on the expression of key markers associated with homeostasis and oxidative stress. A multicenter, open-label clinical study evaluated the efficacy, tolerability, and subject satisfaction of TAP in subjects with photodamaged facial skin over 12 weeks.
Design and Methods
Ex-vivo analysis of key markers. Quantitative real-time polymerase chain reaction (qPCR) analysis was conducted to evaluate gene expression and protein activity of key markers associated with homeostasis and oxidative stress. Donor, full thickness skin tissue was collected within two hours following abdominoplasty. Skin tissue contained normal skin barrier, mature stratum corneum, and functional basal layer, with all cell types and skin appendages of in-vivo human skin. Punch biopises were transferred to static Franz cells. Study products, TAP and L-VC, were applied to skin tissues prior to and following UV radiation (15uL or 30mg/cm2; n=3 each) for a total of 48 hours of contact time with tissues. Tissues were exposed to UV radiation utilizing an Oriel SolA1 Solar Simulator (Newport Corporation; Irvine, California, US) at energy levels comparable to a moderate amount of natural radiation exposure at midday in a temperate climate (250mJ/cm2 of UVA and 0.88mJ/cm2 of UVB). Control tissues were treated with saline (n=3).
Changes in expression of key markers related to epidermal homeostasis and oxidative stress were evaluated. Statistical analysis and changes in gene expression for study products were compared to the untreated control (saline) using an unpaired t-test (p<0.05).
Clinical evaluation. A multicenter, open-label study across three clinical sites enrolled 44 subjects for clinical evaluation following twice-daily application of TAP. Two sites enrolled subjects to undergo evaluation of visible improvements in the appearance of facial photodamage following application of TAP. One site enrolled subjects to undergo biopsy of the left dorsal forearm at baseline and Weeks 6 and 12 following twice-daily application of TAP to both forearms over 12 weeks. Subjects 40 to 65 years of age with Fitzpatrick skin types (FST) I to VI and mild (3) to moderate (6) facial photodamage based on a 10-point grading scale (0=none, 7–9=severe), were eligible for enrollment. Subjects agreed to minimize sun exposure, apply sunscreen daily (15 minutes prior to being outdoors, with reapplication every 2 hours if engaging in outdoor activities), and use only the supplied skincare products. Exclusion criteria included subjects with dermatological disorders (e.g., severe acne vulgaris and/or cystic acne, acne conglobata, acne fulminans, severe rosacea, atopic dermatitis, psoriasis, seborrheic dermatitis), autoimmune diseases, acute illness, or open wounds/lesions or irritated skin in the area to be treated. Subjects who were pregnant, lactating, or planning a pregnancy during the study period were excluded. Subjects were deemed eligible for inclusion in clinical facial evaluations following a two-week washout period of any cosmetic product containing alpha hydroxy acids (AHAs), beta hydroxy acids (BHAs), peptides, growth factors, skin lightening/brightening agents, nonprescription vitamin A derivatives, plant stem cell extracts, vitamin C-based topical antioxidants, and other antioxidants or similar products, and a four-week washout period of products containing prescription retinoids or hydroquinone. Subjects who had taken an oral vitamin A derivative (e.g., isotretinoin) within the previous six months or undergone chemical peels, microdermabrasion, microneedling, or a similar procedure within the previous three months were excluded from study participation.
Subjects enrolled for evaluation of visible improvements in facial photodamage were instructed to apply TAP to the face twice daily and were provided with a standardized regimen consisting of a basic gentle cleanser, moisturizer, and sunscreen (sun protection factor [SPF] 56). Digital images were taken at baseline and Weeks 4, 8, and 12 using Canfield VISIA-CR Imaging System (Canfield Scientific, Inc.; Parsippany, New Jersey, US).
Investigators evaluated changes in the appearance of facial lines/wrinkles, skin texture, skin tone, skin dullness, and erythema from baseline at Weeks 4, 8, and 12 using a 6-point grading scale (0=none, 5=severe). Subjects undergoing evaluation of visible improvements in facial photodamage completed self-assessment questionnaires at each clinic visit. Adverse events (AEs) were collected throughout the study.
A separate group of subjects were enrolled to undergo 3mm circular punch biopsies of the left dorsal forearm at baseline and Weeks 6 and 12 for evaluation of histological changes. Subjects were instructed to apply TAP to both the left and right dorsal forearms from the wrist to the elbow twice daily. TAP was applied to both forearms to maintain symmetry of aesthetic appearance and ensure compliance with product application. Biopsies were taken from the left dorsal forearm, with subsequent biopsies taken approximately one inch from the baseline biopsy location. Histological evaluation and grading of images (0=no staining [target protein], 5=maximal staining [target protein]) occurred at Weeks 6 and 12. Subjects agreed to refrain from the use of moisturizers, lotions, or creams on their forearms for the duration of the study. Institutional Review Board (Advarra; Columbia, Maryland, US) approval was obtained prior to the study. All subjects provided written, informed consent to participate in the study and to have their photographs appear in any publication stemming from the findings of the study. Clinical research sites followed all applicable guidelines in accordance with accepted standards for Good Clinical Practice (GCP).
Results
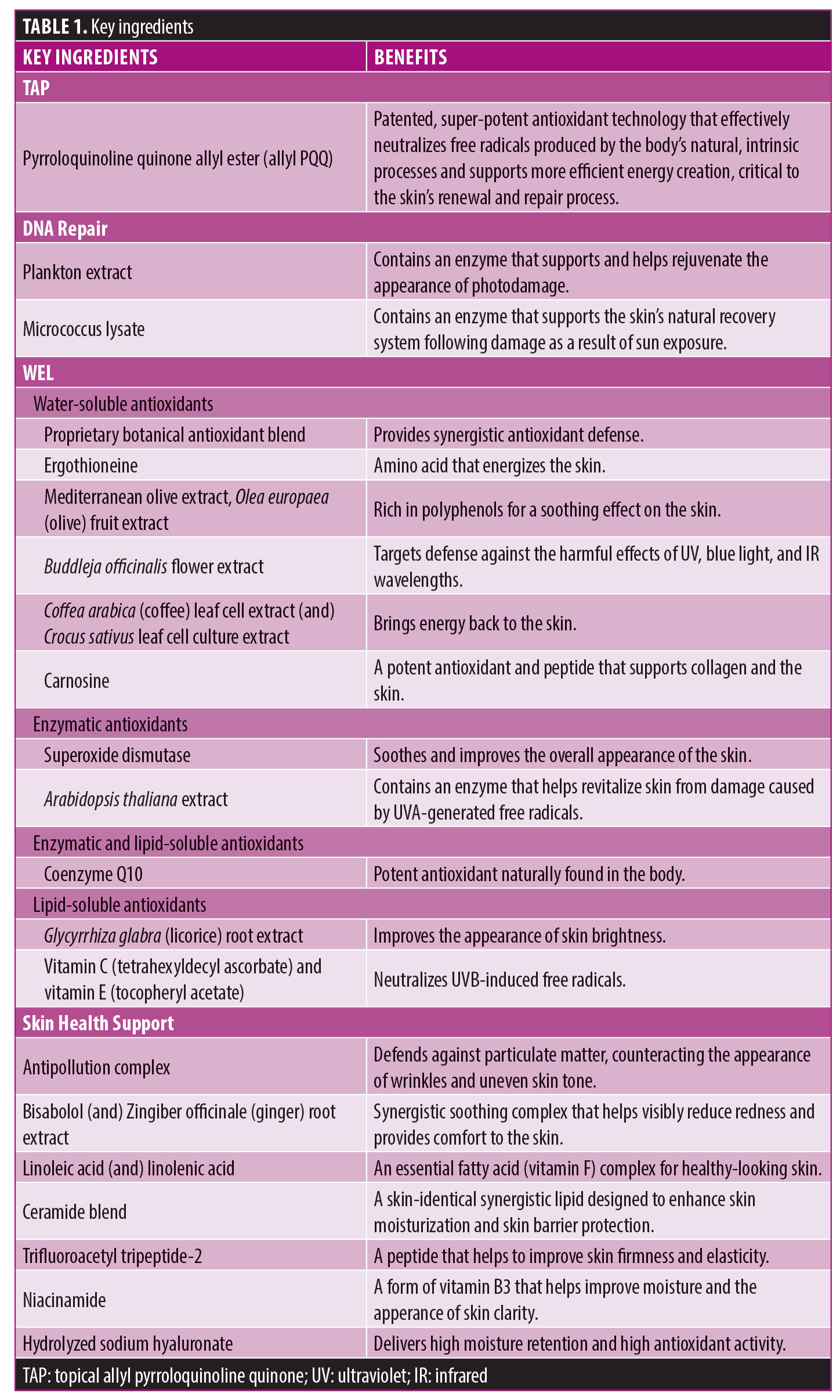
Ex-vivo analysis of key markers. Evaluation of key markers in tissues treated with TAP versus untreated control demonstrated significantly increased expression of DNA methyltransferase (DNMT3A, DNMT3B), cytochrome oxidase assembly factor-10 (COX10), and tumor protein-53 (TP53) genes (all p<0.05), indicating enhanced support of epidermal homeostasis, renewal, and repair. Application of L-VC demonstrated a nonsignificant expression of these markers, compared to untreated control (Figure 1).
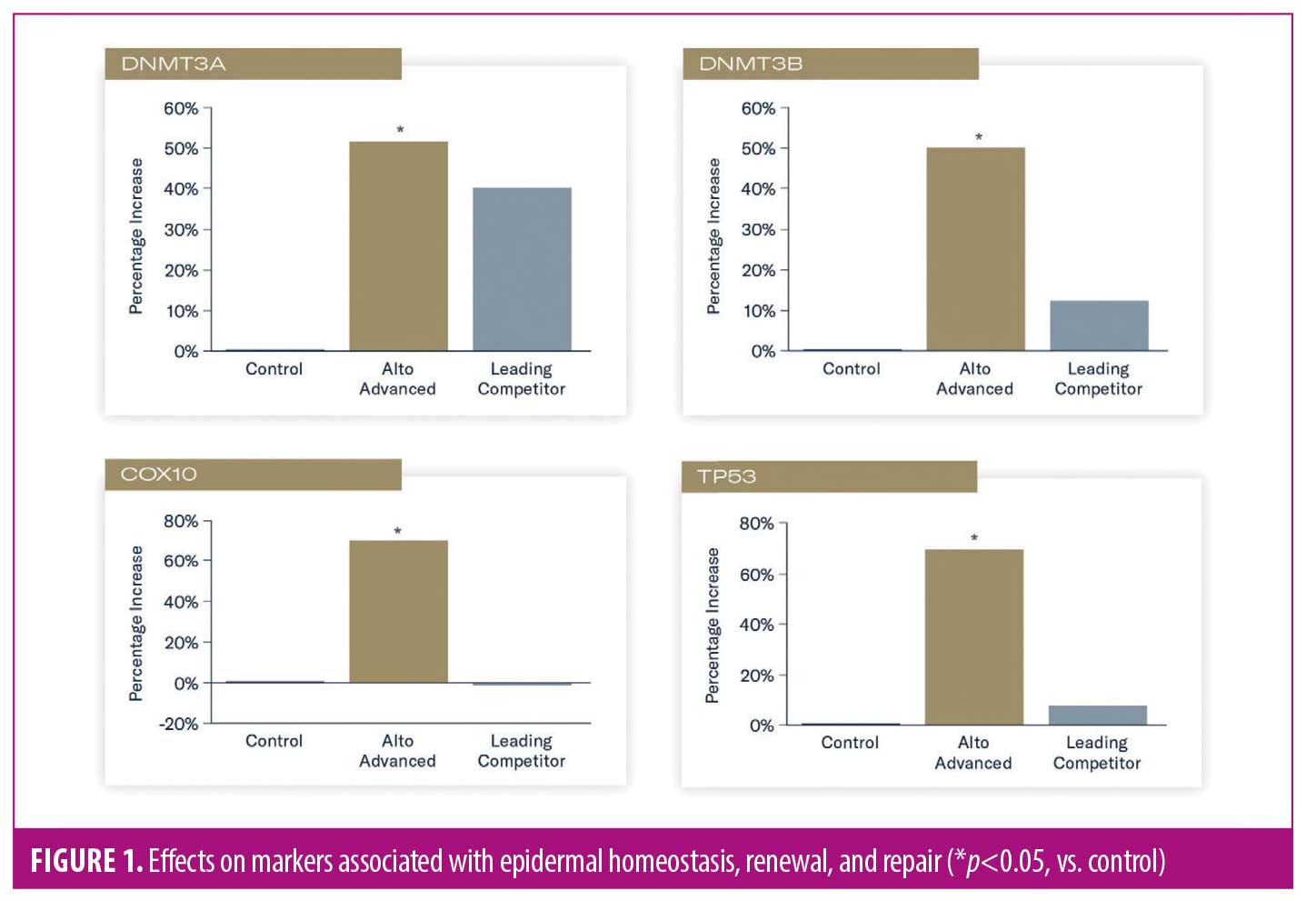
Significantly increased expression of sirtuin-1 (SIRT1) and sequestosome-1/p62 (SQSTM1) were demonstrated in tissues treated with TAP, compared to control (p<0.05), suggesting enhanced oxidative response, protection of collagen from degradation, and autophagy effects. A nonsignificant expression of these markers was demonstrated in tissues treated with L-VC versus control (Figure 2).
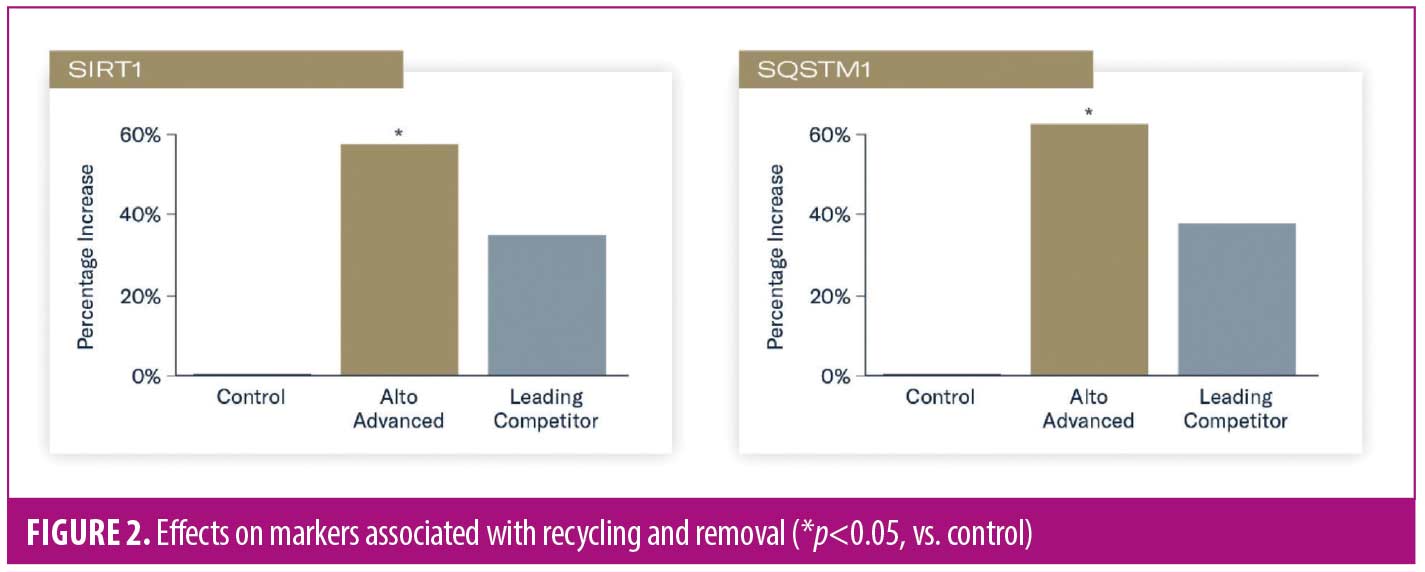
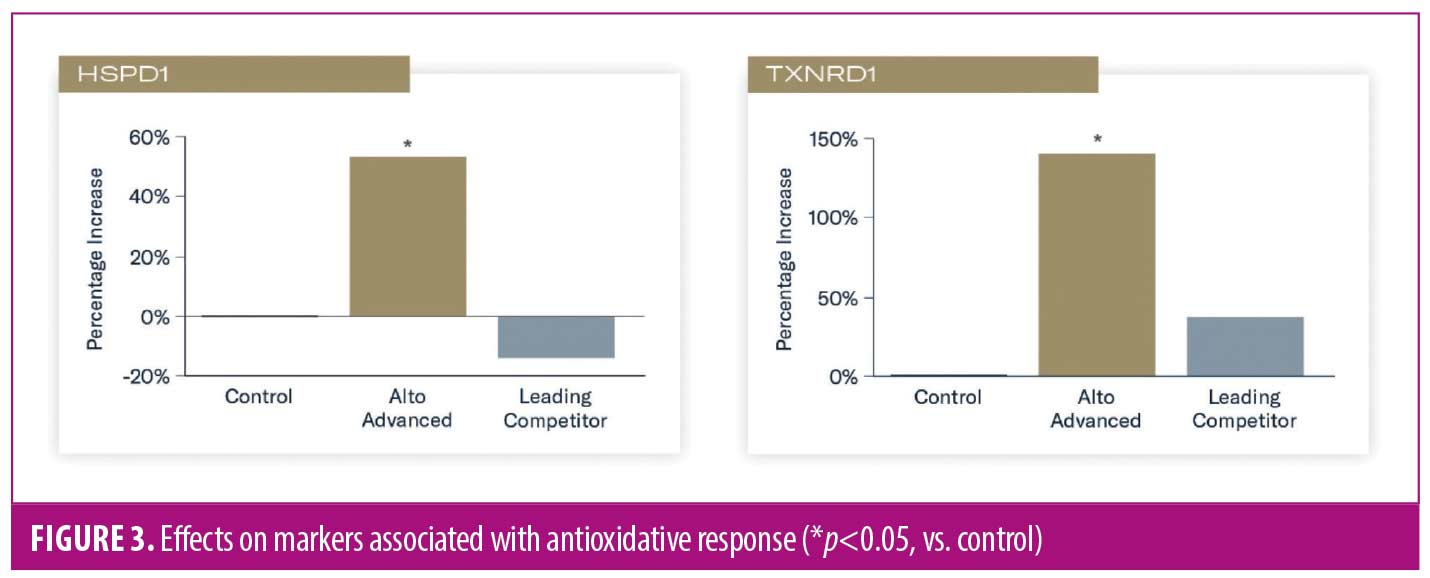
Increased expression of heat shock protein 60 (HSPD1) and thioredoxin reductase (TXNRD1) occurred in tissues treated with TAP versus control (p<0.05), indicating enhanced antioxidative response and adaptation. Applicaton of L-VC demonstrated reduced expression and nonsignificant increases in HSPD1 and TXNRD1, respectively (Figure 3).
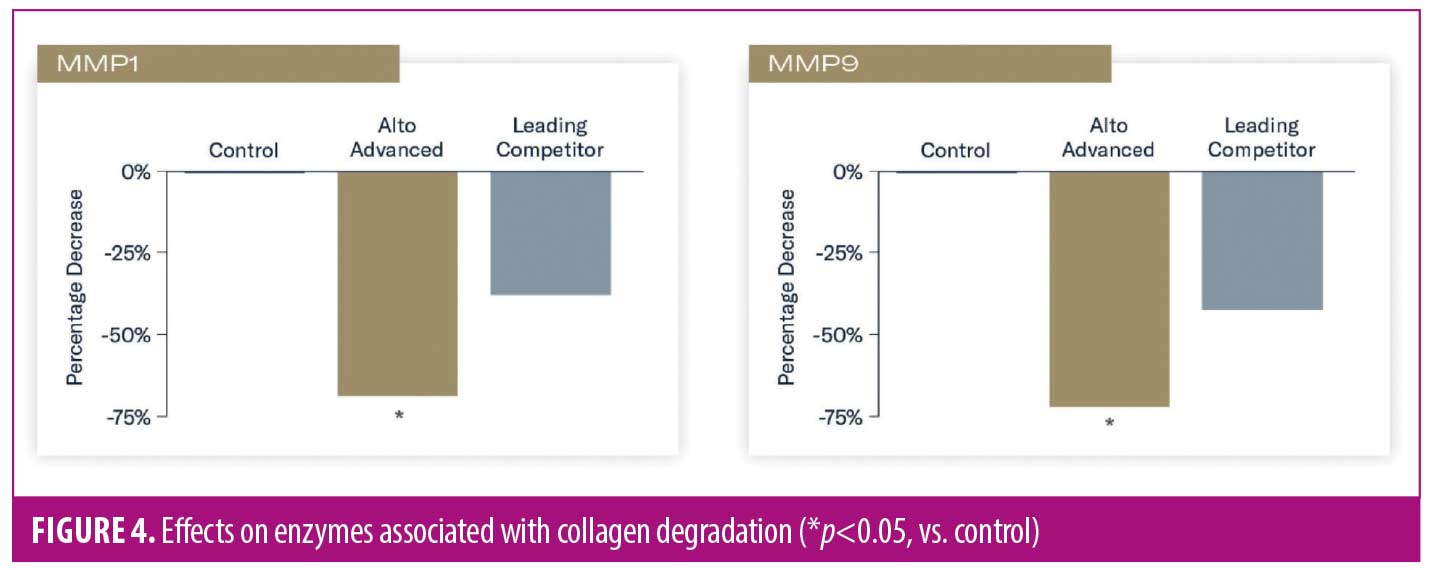
Reduced expression of enzymes associated with the breakdown of collagen types I and III (metalloproteinase-1 [MMP1]) and collagen types II and IV, as well as elastin (matrix metalloproteinase-9 [MMP9]) occurred in tissues treated with TAP versus control (p<0.05). Nonsignificant reductions of these enzymes occurred in tissues treated with L-VC (Figure 4).
Clinical evaluation. Forty-four subjects were enrolled in the study, with 39 subjects completing evaluations of facial photodamage and four subjects undergoing biopsy evaluations. The mean age of enrolled subjects was 54 years, and 82 percent were female. The study population consisted of subjects with FST I (33%), II (46%), III (18%), and IV (3%). The majority of enrolled subjects presented with moderate photodamage (score of 4–6).
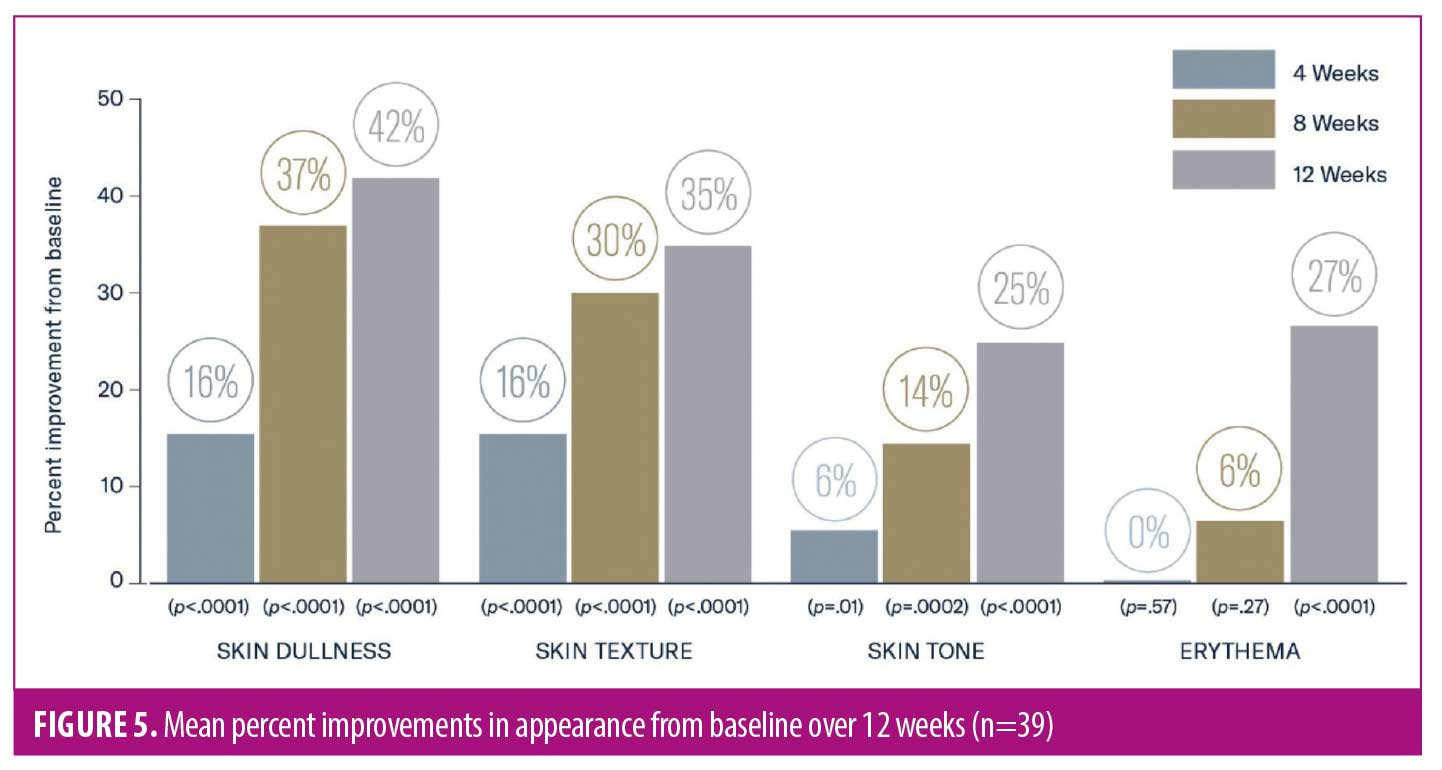
As early as Week 4, significant mean improvements from baseline were observed in skin dullness, skin texture (both p<0.0001), and skin tone (p=0.01). Significant mean improvements were demonstrated from baseline at Week 12 in skin dullness (42%), skin texture (35%), skin tone (25%), and erythema (27%; all p<0.0001) (Figures 5–7). Histologic evaluation demonstrated mean significant improvements in solar elastosis from baseline at Weeks 6 (33%, p=0.01) and 12 (60%, p=0.002) (Figure 8).
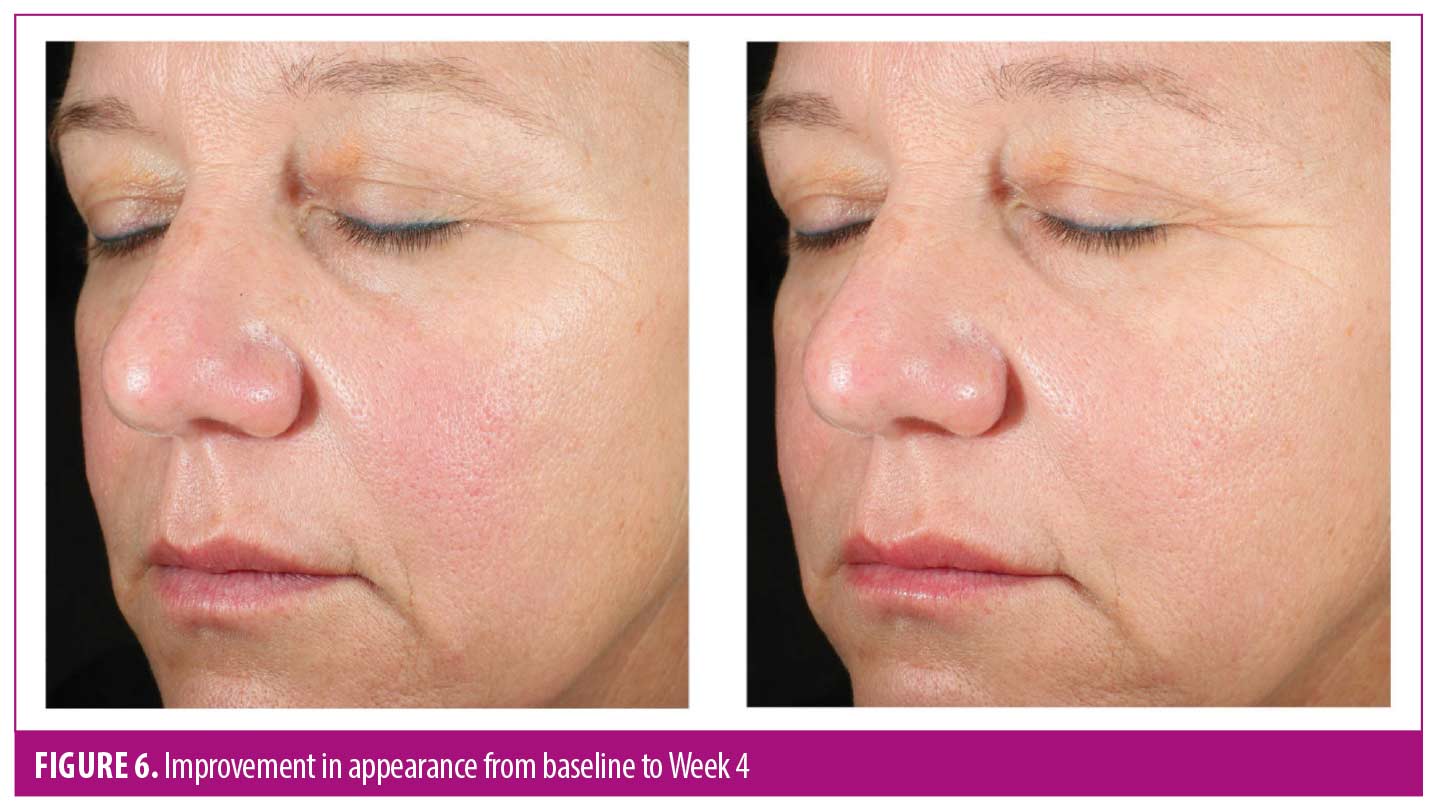
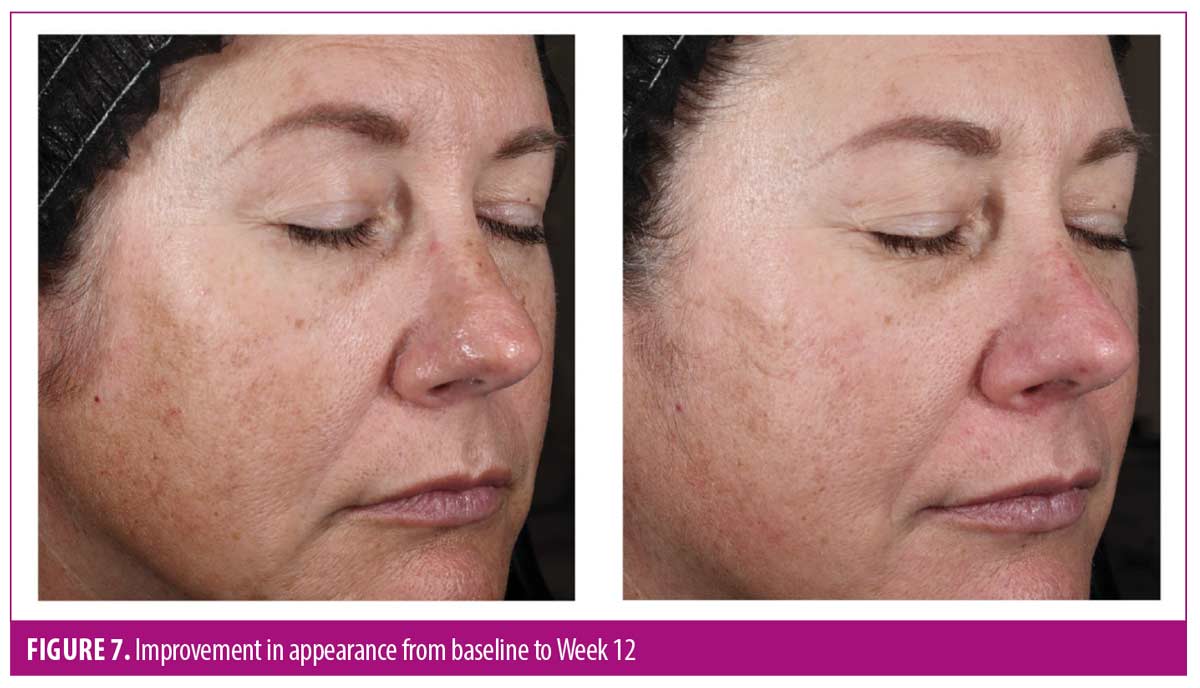
After 12 weeks of use, more than 90 percent of subjects reported overall improvement in the appearance of their skin and brighter, radiant, healthier-looking skin with visible improvement in sun damage. AEs were mild and transient, with one subject reporting moderate, temporary burning sensation upon product contact with the eyes. No subject discontinued the study owing to an AE.
Discussion
Optimal protection of skin from free radical damage should address counteracting ROS generated both intrinsically and extrinsically. Topical antioxidants are a foundational component in supporting skin health. Antioxidants help reduce the formation of free radicals and are valuable in not only preventing DNA damage caused by free radicals, but also in repairing DNA.24 The ability of antioxidants and specific antioxidant combinations to protect skin from external or environmental insults is well established.16,18,25,26 However, less is known regarding the capacity of certain antioxidants in scavenging free radicals generated intrinsically. Mitochondria play a pivotal role in the generation of cellular energy and maintaining homeostasis, and consequently are the primary source of free radicals. As we age, mitochondria become less efficient and more dysfunctional and are degraded by mitophagy. This leads to decreased energy production and an increase in ROS and damage, inducing mitophagy to remove damaged mitochondria. Combined, these factors contribute to intrinsic oxidative stress and accelerated aging. Providing supplemental antioxidant protection and support to mitochondria enables skin to function more efficiently and optimally.
WEL antioxidant technology was formulated using an optimized ratio of 19 potent water-soluble, enzymatic, and lipid-soluble antioxidants to provide broad-based protection for different cellular components of the skin and from a range of free radicals. Many of these antioxidants offer dual benefits to the skin, including reducing inflammation and erythema, brightening skin, and improving the appearance of lines and wrinkles.2,27 Prior research conducted with WEL technology demonstrated its antioxidant capacity in neutralizing hydrogen peroxide in a human skin model.2 A minimal erythema dose (MED) study conducted in healthy subjects demonstrated the ability of WEL technology to protect skin from the oxidizing effects of UVA/B exposure, inducing significantly less erythema and providing substantial cellular protection in comparison to untreated irradiated skin.2 Results from a 12-week clinical trial demonstrated early and substantial improvements in the appearance of fine lines/wrinkles, erythema, and skin tone.2 Additional studies demonstrated WEL’s ability to significantly reduce the effects of ozone-, tobacco-, and blue light-induced oxidative stress in an epidermal skin model and human skin tissue.11,23
This advanced formulation combines WEL technology with TAP technology to provide comprehensive support for skin by targeting both extrinsic and intrinsic causes of damage. Gene expression analysis demonstrated this new formulation’s effect on markers associated with intrinsic skin aging, including epidermal homeostasis, mitochondrial function, autophagy, DNA repair, and oxidative stress.
Additionally, clinical benefits of twice-daily application in subjects with photodamaged skin demonstrated early, significant improvements in the appearance of skin dullness, skin texture, and skin tone with continued, progressive improvements in erythema over 12 weeks. In a small group of subjects, histological evaluation demonstrated improvement in solar elastosis from baseline at Weeks 6 and 12. Subjects reported high levels of satisfaction throughout the study period, with almost all subjects noting an overall improvement in the appearance of their skin and brighter, radiant, healthier-looking skin. The study product was well tolerated, with the subjects reporting only localized, transient AEs.
Limitations. Although conducting mechanistic studies in ex-vivo skin tissue is optimal to understanding activity, results may vary based on individual donors. Moreover, this clinical trial was an open-label study that involved a limited population. Additional clinical research has recently been completed utilizing this new formulation in a diverse group of subjects to help further our understanding of the efficacy and tolerability of TAP across a broader population comprised of a variety of skin types and ethnicities.
Conclusion
Application of a new, advanced antioxidant containing TAP demonstrated a significant expression of key markers associated with epidermal homeostasis and antioxidative response in ex-vivo human skin tissue.
Twice-daily application of the product significantly improved the visible effects of photodamaged skin from baseline over 12 weeks, with histological improvements in solar elastosis demonstrated as early as 6 weeks. Subjects reported high levels of satisfaction with the study product throughout the study period, and the study product was highly tolerable.
These findings demonstrate that an advanced antioxidant incorporating TAP and WEL technologies has the capacity to support skin against the effects of intrinsic and extrinsic oxidative stress and improve the appearance of photodamaged skin.
Acknowledgements
With gratitude for his support and guidance, we would like to acknowledge the contributions of the late Dr. Mitchell Wortzman. We would also like to thank Lynne Kolton Schneider, PhD, for her editorial assistance on this manuscript.
References
- Li J, Liu M, Liang S, et al. Repression of the antioxidant pyrroloquinoline quinone in skin aging induced by Bmi-1 deficiency. BioMed Res Internat. 2022;1732438;1–2.
- McDaniel DH, Waugh JM, Jiang LI, et al. Evaluation of the antioxidant capacity and protective effects of a comprehensive topical antioxidant containing water-soluble, enzymatic, and lipid-soluble antioxidants. J Clin Aesthet Dermatol. 2019;12(4):46–53.
- Sarniak A, Lipińska J, Tytman K, Lipińska S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postepy Hig Med Dosw (Online). 2016;70(0):1150–1165.
- Feichtinger RG, Sperl W, Bauer JW, Kofler B. Mitochondrial dysfunction: a neglected component of skin diseases. Experim Dermatol. 2014;23(9):607–614.
- Stout R, Birch-Machin M. Mitochondria’s role in skin ageing. Biology. 2019;8:29.
- Bowman A, Birch-Machin MA. Age-dependent decrease of mitochondrial complex II activity in human skin fibroblasts. J Investig Dermatol. 2016;136:912–919.
- Singh KK. Mitochondrial secrets of youthfulness. Plast Reconstr Surg. 2021;147(1S-2):33S–37S.
- Balcázar M, Cañizares S, Borja T, et al. Bases for treating skin aging with artificial mitochondrial transfer/transplant (AMT/T). Front Bioeng Biotechnol. 2020;8:919.
- Bielach-Bazyluk A, Zbroch E, Mysliwiec H, et al. Sirtuin 1 and skin: implications in intrinsic and extrinsic aging – a systematic review. Cells. 2021;10(4):813.
- Chistiakov DA, Sobenin IA, Revin VV, et al. Mitochondrial aging and age-related dysfunction of mitochondria. BioMed Res Internat. 2014;Article 238463:1–7.
- Pecorelli A, McDaniel DH, Wortzman M, Nelson DB. Protective effects of a comprehensive topical antioxidant against ozone-induced damage in a reconstructed human skin model. Arch Dermatol Res. 2021;313(3):139–146.
- Sreedhar A, Aguilera-Aguirre L, Singh KK. Mitochondria in skin health, aging, and disease. Cell Death Disease. 2020;11:444.
- Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008;30(2):87–95.
- Zouboulis CC, Makrantonaki E, Mikolakis G. When the skin is in the center of interest: an aging issue. Clin Dermatol. 2019;37(4):296–305.
- Latha MS, Martis J, Shobha V, et al. Sunscreening agents. J Clin Aesthet Dermatol. 2013;6(1):16–26.
- Oresajo C, Stephens T, Hino PD, et al. Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J Cosmet Dermatol. 2008;7(4):290–297.
- Pinnell SR, Yang H, Omar M, et al. Topical L-ascorbic acid: percutaneous absorption studies. Dermatol Surg. 2001;27(2):137–142.
- Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interventions Aging. 2007;2(2):219–236.
- Chowanadisai W, Bauerly KA, Tchaparian E, et al. Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1 α expression. J Biol Chem. 2010;285(1):142–152.
- Misra H-S, Rajpurohit YS, Khairnar NP. Pyrroloquinoline-quinone and its versatile roles in biological processes. J Biosci. 2012;37(2):313–325.
- Jonscher KR, Chowanadisai W, Rucker RB. Pyrroloquinoline-quinone is more than an antioxidant: a vitamin-like accessory factor important in health and disease prevention. Biomolecules. 2021;11(10):1441.
- Sato K, Toriyama M. Effect of pyrroloquinoline quinone (PQQ) on melanogenic protein expression in murine B16 melanoma. J Dermatol Sci. 2009;53(2):140–145.
- Wortzman M, Nelson D. A comprehensive topical antioxidant inhibits oxidative stress induced by blue light exposure and cigarette smoke in human skin tissue. J Cosmet Dermatol. 2021;20(4):1160–1165.
- Draelos ZD. Revisiting the skin health and beauty pyramid: a clinically based guide to selecting topical skincare products. J Drugs Dermatol. 2021;20(6):695–699.
- Grether-Beck S, Marini A, Jaenicke T, Krutmann J. Effective photoprotection of human skin against infrared A radiation by topically applied antioxidants: results from a vehicle controlled, double-blind, randomized study. Photochem Photobiol. 2015;91(1):248–250.
- Mayoral FA, Kenner JR, Draelos ZD. The skin health and beauty pyramid: a clinically based guide to selecting topical skincare products. J Drugs Dermatol. 2014;13(4):414–421.
- Allemann IB, Baumann L. Antioxidants used in skin care formulations. Skin Therapy Lett. 2008;13(7):5–9.

