 J Clin Aesthet Dermatol. 2023;16(1):30-34.
J Clin Aesthet Dermatol. 2023;16(1):30-34.
by Nelva Karmila Jusuf, MD, PhD, Prof; Imam Budi Putra, MD, PhD; and Anggita Dwi Puteri Rangkuti, MD
All authors are with the Department of Dermatology and Venereology, Faculty of Medicine, at Universitas Sumatera Utara, Universitas Sumatera Utara Hospital in Medan, Indonesia
ABSTRACT: Background. Acne vulgaris is a common skin disorder in pilosebaceous units that is self-limited, especially in adolescents. This disease not only causes permanent physical complications but also psychosocial effects that harm the quality of life. Telemedicine has grown its popularity in recent years, especially during the COVID-19 pandemic. Store and Forward (SAF) teledermatology using digital cameras has also increased patient service satisfaction, promising diagnostic reliability, and clinical outcomes similar to face-to-face visits.
Objective. We sought to compare the severity of acne vulgaris by teledermatology with face-to-face consultations. We also observe the capability of teledermatology in establishing the severity of acne vulgaris.
Methods. This study is an observational analytic study with a cross-sectional design involving 105 patients with a diagnosis of acne vulgaris based on inclusion and exclusion criteria. The characteristics of age and sex were recorded. The severity of acne vulgaris was established directly by the resident and teledermatologically by the dermatologist consultant. Teledermatology was carried out based on photo documentation of five facial lesion areas; namely forehead, chin, right cheek, left cheek, and the entire face, along with photos from the history submitted by the resident. An assessment for acne vulgaris severity was carried out based on the classification from the International Consensus Conference on Acne Classification System. This classification divided acne vulgaris as mild, moderate, and severe with an ordinal measuring scale. A compatibility test was also performed to determine the comparison between teledermatology and face-to-face consultations in establishing the severity of acne vulgaris. Comparison of the severity of acne vulgaris was assessed by the kappa value.
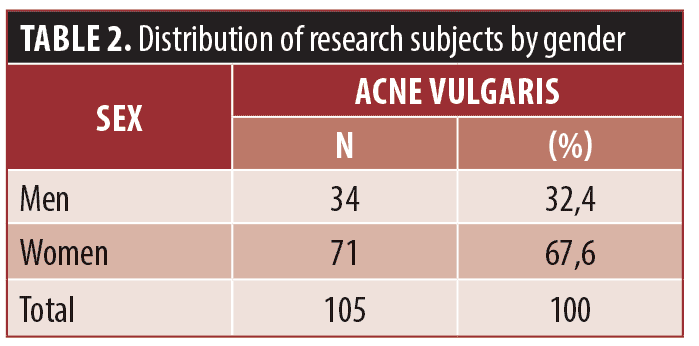
Results. Acne vulgaris was found more common in women (n=71, 67.6%) and those aged 18 to 22 years (n=55, 52.4%). Most of the subjects have moderate severity based on face-to-face consultations and teledermatology examination (n=52 (49.5%) and n=50 (47.6%), respectively). The value of the capability test between teledermatology and face-to-face consultations in comparing the severity of acne vulgaris is 0.611, which means the capability is considered good.
Conclusion. In this study, the teledermatology examination shows good conformity when compared with face-to-face consultations in assessing severity of acne vulgaris.
Keywords: Acne vulgaris, face-to-face consultations, teledermatology, severity of acne vulgaris
Acne vulgaris is a common skin disorder in the pilosebaceous unit that is self-limited, especially in adolescents, both boys and girls.1,2 Acne is most often localized on the face, which plays an essential role in a person’s body image. Acne often interferes with a person’s appearance, causing feelings of displeasure, anxiety, lack of confidence, and social isolation.3
The prevalence of acne vulgaris is 9.4 percent of the global population.4 Data from Indonesia Cosmetic Dermatology Study Group (KSDKI) in 2015 showed that acne vulgaris is a common skin disease in Indonesia. This study group ranked acne vulgaris as the top three visitors to Department of Dermatology and Venereology in Hospital and Dermatology Clinic.5 Data based on previous study at Universitas Sumatera Utara Hospital, stated that the proportion of acne vulgaris incidence from August 2017 to July 2018 is 1.78 percent and the proportion at the period of August 2018 to July 2019 was 1.43 percent.6
Based on patient medical history, acne vulgaris usually occurs at puberty, but the clinical symptoms appear vary widely. The clinical picture of acne vulgaris is in the form of inflammatory lesions and non-inflammatory lesions. The presence of papules, pustules, and nodules characterizes inflammatory lesions, while non-inflammatory lesions are characterized by the presence of comedones (open comedones (blackheads) or closed comedones (whiteheads)).1 Both types of lesions are found in areas with a large number of sebaceous glands.7
Acne vulgaris grading is a subjective method used to determine the severity of acne vulgaris based on the observation of the dominant lesion, evaluation of the presence or absence of inflammatory lesions, and the extent of the skin area involved. The lesion count includes recording the number of each type of acne lesion and determining the overall severity.8,9
The COVID-19 pandemic has limited the care of dermatological patients in many ways.10 Telemedicine has grown in popularity in recent years, and the COVID-19 pandemic has driven telehealth’s widespread use and advancement.11 Teledermatology, as telemedicine in dermatology, can increase the demand for new, more efficient strategies to provide treatment for skin disorders. Cell phones have overcome image resolution limitations seen on older devices, opening up a new field of mobile teledermatology, with approaches of the store and forward (SAF), live video conferencing (LVC), and hybrid. SAF teledermatology allows the transmission of images and text to doctors for examination, while the LVC allows patients and doctors to meet virtually at the same time using a webcam or cell phone camera. Hybrid teledermatology combines both elements of the SAF and LVC methods.12,13
Store and forward using digital cameras has recently succeeded in reducing outpatient waiting times, increasing patient service satisfaction, promising diagnostic reliability, and similar clinical outcomes when compared to face-to-face consultations.14 A systematic review of 21 studies conducted in 2016 reported that the diagnostic accuracy of face-to-face consultations was slightly better (67%-85%, kappa = 0.90) than teledermatology (51%-85%, kappa = 0.41-0,63) in diagnosing skin cancer.15
Other studies have reported teledermatology to be more accurate than face-to-face consultations, possibly due to the increased resolution of cell phone cameras. A total of 391 patients had a diagnosis match between face-to-face consultations and SAF teledermatology (91.05%, (kappa =0.906).16 Our study found there was no significant difference between teledermatology and face-to-face consultations in diagnosing acne vulgaris. Teledermatology can be implemented in the diagnosis of acne vulgaris in daily practice.17
Teledermatology is an alternative that can be easily used for data collection and doctor-patient communication, which provides many benefits for patients.14 This study aimed to compare the capability of teledermatology and face-to-face consultations in assessing the severity of acne vulgaris.
Methods
This study is an observational analytic study with a cross-sectional design conducted at the Dermatology and Venereology Clinic of Universitas Sumatera Utara Hospital, Medan from August 2020 to March 2021.
The subjects of this study were patients aged 18 years who were diagnosed with acne vulgaris and were willing to participate in the study by signing informed consent. Subjects will be excluded if they refuse to take documentation in facial photos using the camera. The sample size in this study was 105 and was recruited using a consecutive sampling method.
The severity of acne vulgaris was assessed directly (face-to-face consultations) by the resident and also teledermatologically by the consultant based on photo documentation of five facial lesion areas along with photos from the history submitted by the authors. The photo areas were captured in the forehead, chin, right cheek, left cheek, and the entire face using a Samsung Galaxy A7-branded cellular phone with a 24MP rear camera. Severity was assessed using the International Consensus Conference on Acne Classification System classification, which consisted of mild (few papules and pustules, no nodules); moderate (a few too many papules and pustules, a few nodules; and severe (there are many extensive papules and pustules, as well as nodules) using an ordinal measuring scale. The collected data was then statistically analyzed by comparing its capability using the kappa statistic according to Cohen’s method.
Results
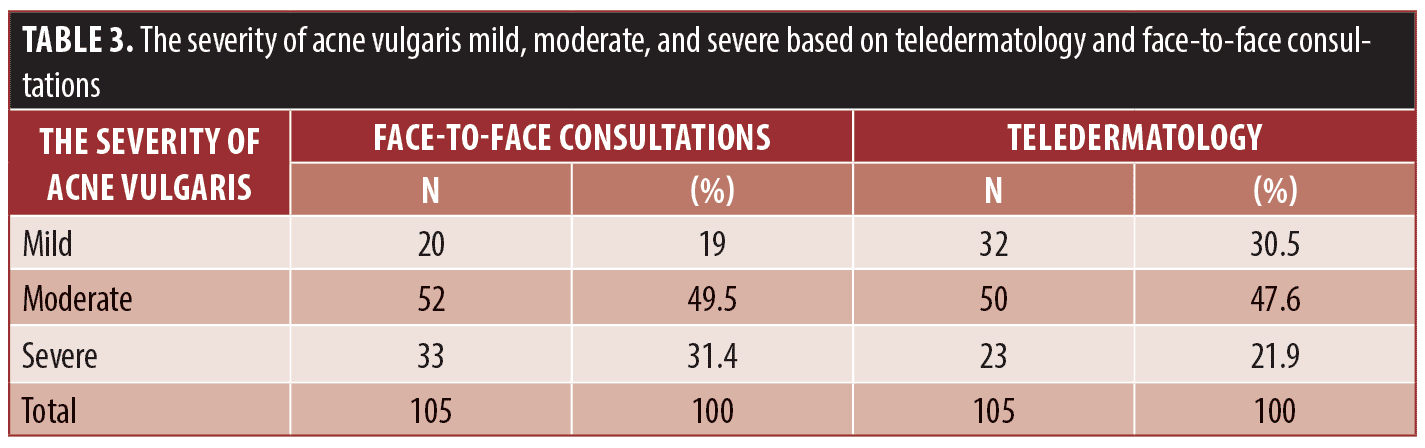
The demographic characteristics of the study subject’s age are described in Table 1. Of the 105 patients who were diagnosed with acne vulgaris, the majority were aged 18 to 22 years old (52.4%), followed by the 23 to 27-year-old subjects (n=29, 27.6%).
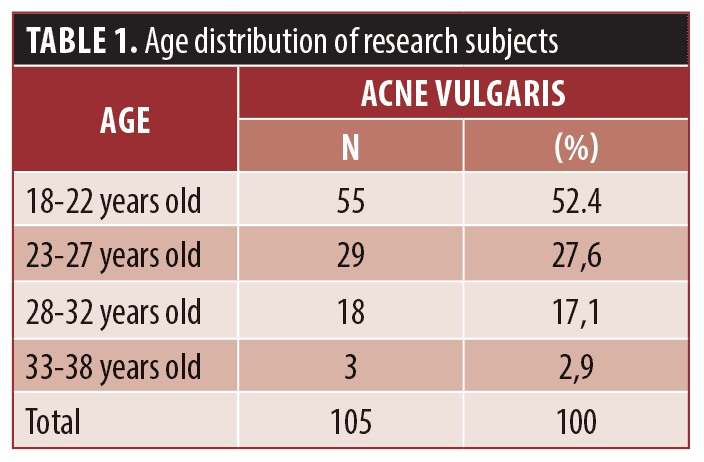
The comparison of the severity of mild, moderate, and severe acne vulgaris based on teledermatology and face-to-face consultations in this study is described in Table 3. We found that the majority have moderate severity when assessed with direct examination (49.5%), followed by severe (31.4%) and mild (19.0%). While on teledermatology examination, the majority also have moderate severity (47.6%) followed by mild (30.5%) and severe (21.9%). This study has found that there are differences in results in mild and severe classification of acne vulgaris.
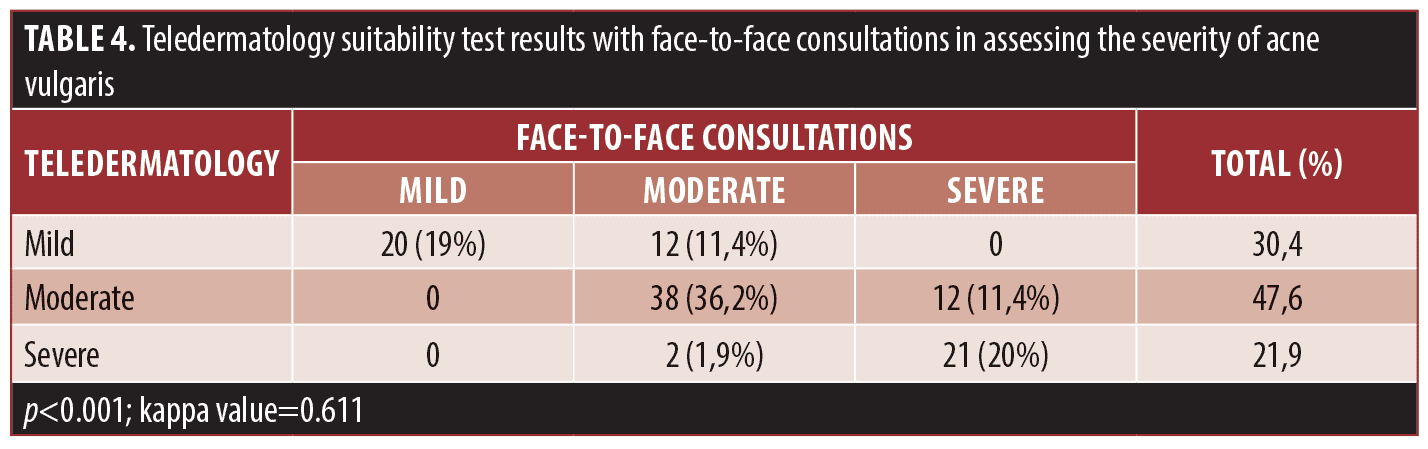
The teledermatology capability test with face-to-face consultations in assessing the severity of acne vulgaris is described in Table 4. We found that 75.2 percent of both examination methods gave same results (concordant cells). By using the kappa test, the p-value <0.001 and the kappa value = 0.611 were obtained. This indicates that the results of the teledermatology examination compared with the results of a face-to-face examination in assessing the severity of acne vulgaris showed good conformity.
Discussion
In the present study, the majority of the subjects were aged 18 to 22 years. This result is similar to a study conducted by Skroza19 in Italy which reported that the majority of acne vulgaris patients were in the 12 to 25 year age range of 58.7 percent. Another study also reported that the prevalence of acne vulgaris patients was mostly at the age of 16 years (89.3%)20 and 15 to 20 years old (37%).22
Most of the subjects in the present study were female (67.%), while the male subjects were 32.4 percent. These results are in line with the study of Alsulaimani et al26 with a prevalence of 90.7 percent in women, but contradict the results of the study of Suppiah et al28 in Malaysia which states that most of the subjects are male (59.6%).
In the present study, most of the subjects were teenagers to young adults. In most cases, acne vulgaris has become a significant problem since the onset of puberty, and its prevalence reaches a peak in mid to late adolescence. The cause of persistent acne vulgaris is unknown. However, several factors are thought to cause this, such as certain cosmetics, drug consumption, and endocrine disorders. Specific individuals are said to have increased androgen levels causing persistent acne vulgaris.23 Androgens have always been involved in the pathogenesis of acne vulgaris.24
According to the researchers, the differences in the prevalence of acne vulgaris based on gender were due to variations in the research sample involved in each study. Hormonal factors are thought to be one of the causes of the high prevalence of acne vulgaris in women compared to men. This is because the onset of puberty in women occurs earlier than in men, and acne vulgaris tends to be more persistent in women.1,29 Other factors that are thought to influence the incidence are the use of cosmetics and the tendency of women to seek treatment immediately when experiencing cosmetic complaints.30
Apart from those mentioned above, there are several other factors that affect the severity of acne. A study by Sutrisno et al31 concluded that there is a positive correlation between stress levels and acne severity. An increase in sebum production that triggers an increase in CRH when stressed is thought to cause this. Stress also stimulates neurogenic inflammation, which increases the production of pro-inflammatory cytokines and results in the proliferation, differentiation, and lipogenesis of the pilosebaceous glands.
Another study by Jusuf et al32 stated that there was a significant positive correlation between stress levels and serum substance P levels in acne vulgaris, with moderate strength. The higher the stress level, the higher the level of substance P. Substance P is sent by peripheral nerves in response to stress. Substance P can stimulate the proliferation of sebaceous precursor cells and increase the size of sebocytes. So that there will be proliferation and differentiation of the sebaceous glands and an increase in lipid synthesis in the sebocytes.32
Skin type also has a significant relationship with acne vulgaris. The study of Tambe et al33 stated that as many as 67.7 percent of subjects with acne had a skin type like. Increased sebum production may be influenced by hormones. The higher the androgen hormone, the higher the sebum production because the androgen hormone is thought to affect the keratinization process. Increased sebum production and abnormal proliferation of keratinocytes can lead to ductal obstruction and primary acne lesions as microcomedoes. On the other hand, increased sebum can lead to colonization of C. acnes which in turn exacerbates acne vulgaris.33
Another factor that affects the severity of acne is the level of skin hydration. The study of Yolanda et al34 stated that the lower the level of hydration of the facial skin, the more severe the degree of acne vulgaris. Also, decreased phytosphingosine, which plays an important role in skin permeability and antimicrobial defense, can cause changes in the skin barrier, increase inflammation and infection, and impair the skin’s water barrier function. Decreased skin hydration levels, chapped skin, and desquamation clinically will cause changes in skin barrier function, facilitating the invasion of pathogens in the pilosebaceous unit which have been shown to cause an inflammatory reaction.34
Body Mass Index (BMI) is another factor that affects the severity of acne. An analytical observational study by Deliana et al35 concluded that students with a BMI in the obese category had a 1.438 times greater risk of acne vulgaris compared to students in the non-obese BMI category. Obesity affects hyperandrogenism through increased IGF-1 which causes follicular hyperkeratinization and sebaceous glands to secrete sebum production. Increased sebum production and follicular hyperkeratinization will trigger the formation of acne vulgaris.35
In the present study, the assessment of moderate acne vulgaris is similar between teledermatology and face-to-face consultation. There are differences in the assessment of mild and severe acne vulgaris through teledermatology and face-to-face consultations. This discrepancy could be due to previously reported factors, such as the quality of the images transmitted by teledermatology consultation. However, there is no general imaging standard that has been developed and implemented in teledermatology until now.36,37
Guidelines by the American Telemedicine Association (2008)38 mention that a 24-bit image that offers 16,777,216 color values is needed to perform teledermatology. Other literature mentions that the resolution of 768 × 512 pixels is also suitable for teledermatology. The American Telemedicine Association’s (2012) guideline recommends a minimum of 800 × 600 pixels and prefers a resolution of 1024 × 768 pixels for SAF teledermatology.38
Another factor is the quality of the images taken from the cell phone camera is not the same as with a digital camera. The image quality of a cell phone camera may be close to a digital camera in terms of the number of megapixels but not in sensor size. For example, the average sensor on a 10-megapixel cell phone camera produces much lower resolution images than a 10-megapixel single-lens reflex (SLR) digital camera sensor. In addition, there is the problem of limited space in mobile phones to accommodate other components such as lenses, apertures (lens holes), and flashes.4
Another major drawback is the absence or inadequate optical zoom on the cell phone camera. In special digital cameras, lesions can be enlarged using optical zoom for close-up images. Taking photos that are too close to the lesion tends to produce spherical distortion, which may look odd, especially on facial photographs.39
Lighting and patient positioning are big concerns in terms of ensuring image consistency for follow-up documentation. Particular areas of the clinic with fixed lighting conditions are ideal for clinical photography. In general, it is better to use flash (if available) all the time as it tends to reduce image blur.40
Another reason for differences in assessing mild and severe acne vulgaris through teledermatology with FTF consultations in this study could be photo documentation sent via the WhatsApp application. According to Anwar et al,41 research on the analysis of Portable Network Graphics (PNG) image quality on delivery via the WhatsApp application, images sent directly without being converted into documents will be compressed directly by the application will achieve maximum dimensional resolution by paying attention to the dimension ratio. Simultaneously, image dimensions less than the maximum dimensions will not be reduced in dimensions (only the file size value will be reduced).41 This possibility may lead to a change in the lesion’s shape from the patient’s photograph when the supervisor received the photo documentation for this study.
The kappa obtained in this study was 0.611. This value indicates that the results of the teledermatology examination compared with the results of face-to-face consultations in assessing the severity of acne vulgaris showed good conformity. Teledermatology capability test is still limited. According to the research report of Oliveira et al42 in Brazil, the results of the diagnostic capability varied between teledermatology and face-to-face consultations, ranging from a kappa value of 0.35 to almost perfect with 0.91. However, most studies show a near-perfect fit. Studies using a teledermatology strategy have shown benefits in improving skin condition severity, adherence to therapy, and higher quality of life than usual care. Overall, the intervention results showed the same clinical improvement when compared to the usual care.42
Conclusion
The capability between teledermatology and face-to-face consultations showed good conformity in assessing severity of acne vulgaris. Therefore teledermatology is recommended in establishing severity of acne vulgaris if face-to-face consultation can not be done.
Limitations. Limitations included some technical issues and the impossibility to suggest how soon the patient should be assisted face-to-face by a dermatologist. And the limitation of this research is that there is no official software/platform available.
References
- Goh C, Cheng C, Agak G, et al. Acne vulgaris. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, Orringer JS, editor. Fitzpatricks’s Dermatology in General Medicine. 9th Ed. New York: The McGraw Hill Companies. 2019; p1391–1418.
- James WD, Elston DM, Berger TG, et al. Andrews’ Diseases of the Skin: Clinical dermatology. London: Saunders/Elsevier. 2020; pp 231.
- Hanstock TL, O’Mahony JF. Perfectionism, acne and appearance concerns. Personality and Individual Differences. 2002; 32(8): 1317–1325.
- Tan JK, Bhate K. A global perspective on the epidemiology of acne. British Journal of Dermatology. 2015; 172: 3–12.
- Wasitaatmadja SM, Arimuko A, Norawati L, et al. Pedoman tata laksana akne di Indonesia. 2th Ed. Jakarta: Centra communications. 2016: 1–16.
- Marpaung SC, Jusuf NK, Putra IB. Studi Retrospektif Akne Vulgaris Tahun. 2017-2019 di RS Universitas Sumatera Utara Medan.
- Batra, Sonia. Acne. Arndt KA, Hs JT, eds. Manual of Dermatology Therapeutics. 7th ed. Massachusetts: Lippincott Williams and Wilkins. 2007.
- Adityan B, Kumari R, Thappa DM. Scoring Systems in Acne Vulgaris. Indian J Dermatol Venereol Leprol. 2009; 75: 323–326.
- Witkowski JA, Parish LC. The Assessment of Acne: An Evaluation of Grading and Lesion Counting in the Measurement of Acne. Clinics in Dermatology. 2004; 23: 394–397.
- Elsner P. Teledermatology in the times of COVID‐19– a systematic review. JDDG: Journal Der Deutschen Dermatologischen Gesellschaft. 2020; 18(8): 841–845.
- Rustad AM, Lio PA. Pandemic Pressure: Teledermatology and Health Care Disparities. Journal of Patient Experience. 2021; 8: 1–5.
- Brinker TJ, Hekler A, Kalle CV, et al. Teledermatology: Comparison of Store-and-Forward Versus Live Interactive Video Conferencing. J Med Internet Res. 2018; 20(10): e11871.
- Bashshur RL, Shannon GW, Tejasvi T. The Empirical Foundations of Teledermatology: A Review of the Research Evidence. Telemedicine and e-Health. 2015; 21(12): 953–979.
- Tran K, Ayad M, Weinberg J, et al. Mobile teledermatology in the developing world: Implications of a feasibility study on 30 Egyptian patients with common skin diseases. Journal of the American Academy of Dermatology. 2011; 64(2): 302–309.
- Finnane A, Dallest K, Janda M, et al. Teledermatology for the Diagnosis and Management of Skin Cancer. JAMA Dermatology. 2017;153(3): 319.
- Nami N, Massone C, Rubegni P, et al. Concordance and time estimation of store-and-forward mobile teledermatology compared to classical face-to-face consultation. Acta Derm Venereol. 2015; 95(1): 35–39.
- Rangkuti ADP, Jusuf NK, Putra IB. Comparison between the diagnosis of acne vulgaris by teledermatology and face-to-face consultations. Bali Medical Journal. 2021;10(3): 1037–1040.
- Collier CN, Harper JC, Cantrell WC, et al. The prevalence of acne in adults 20 years and older. Journal of the American Academy of Dermatology. 2008;58(1): 56–59.
- Skroza N, Tulino E, Mambrin A, et al. Adult Acne Versus Adolescent Acne: A Retrospective Study of 1.167 Patients. Journal of Clinical and Aesthetic Dermatology. 2018;11(1):21–25.
- Vilar GN, Santos LA dos, Sobral Filho JF. Quality of life, self-esteem and psychosocial factors in adolescents with acne vulgaris. Anais Brasileiros de Dermatologia. 2015;90(5):622–629.
- Perera MPN, Peiris WMDM, Pathmanathan D. Relationship between acne vulgaris and cosmetic usage in Sri Lankan urban adolescent females. Journal of Cosmetic Dermatology. 2017;17(3):431–436.
- Alanazi TM, Alajroush W, Alharthi RM, et al. Prevalence of Acne Vulgaris, Its Contributing Factors, and Treatment Satisfaction Among the Saudi Population in Riyadh, Saudi Arabia: A Cross-sectional Study. Journal of Dermatology and Dermatologic Surgery. 2020; 24:33–37. 10.232.74.23.
- Williams C, Layton AM. Persistent Acne in Women. American Journal of Clinical Dermatology. 2006;7(5):281–290.
- Bhambri S, Del Rosso JQ, Bhambri A. Pathogenesis of acne vulgaris: recent advances. J Drugs Dermatol. 2009;8(7):615–618.
- El-Hamd MA, Nada EE-DA, Moustafa MA-K, et al. Prevalence of acne vulgaris and its impact of the quality of life among secondary school-aged adolescents in Sohag Province, Upper Egypt. Journal of Cosmetic Dermatology. 2017;16(3):370–373.
- Alsulaimani H, Kokandi A, Khawandanh S, et al. Severity of Acne Vulgaris: Comparison of Two Assessment Methods. Clinical, Cosmetic and Investigational Dermatology. 2020;13:711–716.
- Wolkenstein P, Machovcová A, Szepietowski JC, et al. Acne prevalence and associations with lifestyle: a crosssectional online survey of adolescents/young adults in 7 European countries. Journal of the European Academy of Dermatology and Venereology. 2017;32(2):298–306.
- Suppiah TSS, Sundram TKM, Tan ESS, et al. Acne Vulgaris and its Association with Dietary Intake: a Malaysian Perspective. Asia Pac J Clin Nutr. 2018;27(5):1141–1145.
- Baumann L, Keri J. Acne (type 1 sensitive skin). In: Baumann L, Saghari S, Weissberg E, (eds.) Cosmetic Dermatology Principles and Practice. Edisi 2. New York: McGraw Hill. 2009: p.121–127.
- Khunger N, Kumar C. A clinico-epidemiological study of adult acne: Is it different from adolescent acne? Indian Journal of Dermatology, Venereology, and Leprology. 2012; 78(3):335.
- Sutrisno AR, Jusuf NK, Putra IB. Correlation between Stress Scale and Severity of Acne Vulgaris. Bali Medical Journal. 2020; 9(1):376–379.
- Jusuf NK, Putra IB, Angie RS. Correlation Between Stress Scale and Serum Substance P Level in Acne Vulgaris. International Journal of General Medicine. 2021;14: 681–686.
- Tamba ABP, Jusuf NK. The Association Between Skin Types and Acne Vulgaris. Sumatera Medical Journal. 2020;3(1):34–40.
- Yolanda MO, Jusuf NK, Putra IB. Lower facial skin hydration level increases acne vulgaris severity level. Bali Medical Journal. 2021;10(3): 1081–1084.
- Deliana R, Amalia R, Jusuf NK. Hubungan Indeks Massa Tubuh dengan Akne Vulgaris pada Siswa-Siswi SMA Negeri 7 Medan. CDK. 2019;46(4):253–255.
- Legiawati L. Terapi rumatan dan adjuvan pada akne vulgaris, dalam: Wasitaatmadja SM, editor. Akne. Jakarta: Badan Penerbit Fakultas Kedokteran Universitas Indonesia. 2018, h.145–152.
- McKoy K, Antoniotti NM, Armstrong A, et al. Practice Guidelines for Teledermatology. Telemedicine and e-Health. 2016; 22(12): 981–990.
- Tensen E, Heijden JP, Jaspers MWM, et al. Two Decades of Teledermatology: Current Status and Integration in National Healthcare Systems. Current Dermatology Reports. 2016; 5(2):96–104.
- Ashique K, Aurangabadkar S, Kaliyadan F. Clinical photography in dermatology using smartphones: An overview. Indian Dermatology Online Journal. 2015;6(3):158.
- Kaliyadan F, Amin TT, Kuruvilla J, et al. Mobile teledermatology-patient satisfaction, diagnostic and management concordance, and factors affecting patient refusal to participate in Saudi Arabia. Journal of Telemedicine and Telecare. 2013; 19(6):315–319.
- Anwar F, Fadlil A, Riadi I. Image Quality Analysis of PNG Images on WhatsApp Messenger Sending. Telematika. 2021; 14(1):1–12.
- Oliveira JA, Wolff IS, Ribeiro LD, et al. Teledermatology: current perspectives. Smart Homecare Technology and TeleHealth. 2017;4: 61–73.

